ABSTRACT
- Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, characterized by an irregular and rapid beating of the atria, which results in a loss of effective atrial contraction. The estimated prevalence of AF in the general population is approximately 0.4%. Research on the incidence of AF indicates a significant increase with age. AF presents a significantly higher risk of stroke compared to normal sinus rhythm, with the risk increasing approximately fivefold. It is estimated that around 5% of AF patients suffer a stroke annually. Roughly 20% to 25% of thromboembolic strokes can be attributed to AF, and AF is also associated with a twofold increase in overall mortality. The goals of AF treatment are symptom relief, restoration of normal cardiac function, prevention of thromboembolism, and reduction in mortality. Therefore, the treatment principles can be summarized into three categories: thromboembolism prevention, rate control, and rhythm control. In the treatment of AF, the first step should be to identify and eliminate any underlying causes or triggers. Caution should be exercised regarding the potential for drug-induced arrhythmias or extracardiac side effects. Safety considerations should take precedence over efficacy when selecting antiarrhythmic drugs. Nonpharmacological treatment methods are employed when anti-arrhythmic drug therapy alone is insufficient, particularly in relatively young individuals (under 70 years) without preexisting heart disease, who have experienced frequent transitions from atrial premature contractions or AF instigated by atrial premature contractions. Monitoring the patient's progress is vital, with a focus on comprehensive care for patients with AF.
-
Keywords: Atrial fibrillation; Anticoagulation; Catheter ablation; Surgery
ATRIAL FIBRILLATION
- Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, characterized by an irregular and rapid beating of the atria, which results in a loss of effective atrial contraction. On an electrocardiogram, AF is recognized by a notably fast and irregular atrial rhythm, with atrial rates fluctuating between 350 and 600 beats/min and a highly irregular ventricular response, also known as an irregularly irregular pulse.
- The estimated prevalence of AF in the general population is approximately 0.4%. Research on the incidence of AF indicates a significant increase with age. The prevalence is less than 1% in individuals under 60 years old, around 1% in those over 60 years, 2% in individuals in their 70s, and 6% in those aged 80 years and above [1].
DIAGNOSIS
- Paroxysmal AF spontaneously resolves and recurs sporadically. Persistent AF is diagnosed when AF persists for more than one week or could be converted back to a normal rhythm using antiarrhythmic medications or electrical cardioversion. Permanent AF, on the other hand, is characterized by AF that, once initiated, continues indefinitely, or AF that is accepted by the patient and physician, and no further attempts to restore sinus rhythm will be undertaken. In clinical practice, AF is classified as paroxysmal if it naturally resolves within a week, and as persistent if it continues beyond a week without spontaneously resolving. The distinction between persistent and permanent AF can sometimes be unclear, leading to the classification of chronic AF for longstanding persistent AF that has lasted for more than 1 year. Recurrent AF is defined as two or more episodes of AF. Approximately 10% of paroxysmal AF cases progress to persistent or chronic AF within a year, with a tendency to become increasingly chronic over time. The term "lone AF" is used when AF occurs in individuals under 60 years who show no clinical or echocardiographic evidence of specific diseases such as hypertension or heart failure [1].
CAUSES
- Paroxysmal AF often occurs without underlying heart disease and can be triggered by factors such as mental stress, intense exercise, surgery, acute alcohol intoxication, and autonomic nervous system abnormalities. In contrast, persistent AF is commonly linked with conditions such as hypertension, valvular heart disease, myocardial ischemia, cardiomyopathy, heart failure, congenital heart disease, and chronic lung disease. Hyperthyroidism is a prevalent cause of initial-onset AF. The prevalence of AF increases with the severity of heart failure, with approximately 10% in New York Heart Association (NYHA) class I and up to 50% in NYHA class IV heart failure [1].
SYMPTOMS
- The symptoms of AF are as follows. First, a rapid ventricular heart rate can lead to low blood pressure and pulmonary congestion, accompanied by palpitations and anxiety. Second, syncopal episodes and dizziness may occur due to sinus pause immediately after the termination of AF. Third, systemic embolism, such as stroke, can be a consequence. Fourth, general fatigue and shortness of breath may accompany a decrease in cardiac output due to the loss of atrial contractions. Fifth, symptoms related to AF may range from none to disabling.
- Especially when cardiac function is significantly impaired, the combination of a rapid ventricular response and the loss of atrial contraction function can result in ineffective ventricular filling with blood. This can lead to hemodynamic instability, manifesting as low blood pressure, syncope, heart failure, and tachycardia-induced cardiomyopathy. These conditions can be triggered simply by the presence of AF [1].
COMPLICATIONS
- AF presents a significantly higher risk of stroke compared to normal sinus rhythm, with the risk increasing approximately fivefold. It is estimated that around 5% of AF patients suffer a stroke annually. Roughly 20% to 25% of thromboembolic strokes can be attributed to AF, and AF is also associated with a twofold increase in overall mortality [1].
- In an autopsy study of AF patients 70 years or older, cerebral infarctions were present in 60% and cardioembolic infarctions were present in 65% of AF patients with symptomatic cerebral infactions [2]. These instances often result in more extensive brain damage and severe neurological impairment, leading to a higher risk of death or severe disability compared to strokes caused by other factors. Consequently, for patients with AF, preventing thromboembolism through anticoagulation therapy is deemed the most crucial aspect of the treatment strategy.
RISK OF STROKE (THE CHA2DS2-VASc SCORE)
- The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category [female]) score is a comprehensive tool used to evaluate the risk of stroke in patients with non-valvular AF, which refers to AF without moderate to severe mitral stenosis or a mechanical prosthetic mitral valve. This score considers several factors including heart failure, hypertension, age (with 2 points assigned for age 75 years and older, and 1 point for age 65–74 years), diabetes, previous stroke (2 points), vascular disease, and sex (with the female sex receiving 1 point). Patients with a risk score of 2 or more are classified as high risk for stroke, and anticoagulation therapy is recommended. Even for patients with a risk score of 1, anticoagulation therapy may be considered, but only after weighing the risk of stroke against the risk of bleeding [1].
PRINCIPLES OF AF TREATMENT
- The goals of AF treatment are symptom relief, restoration of normal cardiac function, prevention of thromboembolism, and reduction in mortality. Therefore, the treatment principles can be summarized into three categories: thromboembolism prevention, rate control, and rhythm control.
- In the treatment of AF, the first step should be to identify and eliminate any underlying causes or triggers. If there is hemodynamic instability or ongoing myocardial ischemia, as seen in cases of low blood pressure or shock, direct-current cardioversion should be the initial treatment option.
ANTICOAGULATION TREATMENT
- To prevent thromboembolism resulting from AF, it is necessary to tailor anticoagulation drug therapy, using either warfarin or nonvitamin K oral anticoagulants (NOACs), according to each patient's individual risk profile [1].
- Warfarin has traditionally been recommended for stroke prevention in AF, with a target international normalized ratio (INR) range of 2.0 to 3.0. Regular blood tests are necessary to monitor these INR levels. However, in real-world clinical scenarios, less than half of patients are able to maintain an INR within this range. The use of warfarin often presents challenges due to drug interactions and dietary restrictions, in addition to the inconvenience of regular blood tests. As a result, NOACs such as dabigatran, rivaroxaban, apixaban, and edoxaban, were developed and now are widely used in clinical practice. Large-scale domestic and international clinical studies have shown that these NOACs are effective in preventing strokes, and they even outperform warfarin in reducing the risk of cerebral hemorrhage (Table 1).
PRINCIPLES OF ANTIARRHYTHMIC DRUG USE
- The principles of antiarryhtmic drug use are the following.
- (1) The goal of treatment is to alleviate the symptoms related to AF.
- (2) Antiarrhythmic drugs aimed at maintaining normal sinus rhythm typically exert moderate efficacy.
- (3) Clinically, antiarrhythmic drug therapy tends to reduce AF, rather than completely eliminating it.
- (4) When one antiarrhythmic drug fails, trying another medication may achieve clinically satisfactory results.
- (5) Caution should be exercised regarding the potential for drug-induced arrhythmias or extracardiac side effects.
- (6) Safety considerations should take precedence over efficacy when selecting antiarrhythmic drugs.
- Side effects can occur when using antiarrhythmic drugs (Table 2). Thus, if asymptomatic AF is incidentally discovered without accompanying symptoms such as heart failure, palpitations, or dizziness—particularly in instances of longstanding persistent AF—only anticoagulation therapy may be considered for stroke prevention, provided the heart rate is normal. The patient's condition can then be closely monitored.
- Medications typically used for cardioversion are often class I or III antiarrhythmic drugs. For patients without structural heart disease, primary choices include class Ic drugs such as flecainide, pilsicainide, and propafenone. Conversely, for patients with structural heart disease, amiodarone and sotalol are more appropriate options (Table 2, Fig. 1). However, the effectiveness of these antiarrhythmic drugs is generally limited. This is especially true in the case of persistent AF, where the success rate is less than 50%. While amiodarone is known for its higher efficacy, it can also lead to side effects such as lung toxicity, thyroid toxicity, and proarrhythmic problems.
NONPHARMACOLOGICAL TREATMENT
- Antiarrhythmic drugs are used with the goal of suppressing the occurrence and recurrence of arrhythmias, rather than curing them outright. Consequently, they often require long-term use, which increases the risk of side effects and incurs significant costs. However, due to the limited efficacy of antiarrhythmic drugs, efforts to find nonpharmacological treatment methods for AF have been accelerating.
- A critical mechanism underlying the onset of AF involves the pulmonary veins and atrial remodeling. Nonpharmacological interventions, such as percutaneous catheter ablations and surgical procedures, have shown superior outcomes compared to pharmacological approaches. This has led to the widespread adoption of these nonpharmacological treatments worldwide.
- Nonpharmacological treatment methods are employed when antiarrhythmic drug therapy alone is insufficient, particularly in relatively young individuals (under 70 years) without preexisting heart disease, who have experienced frequent transitions from atrial premature contractions or AF instigated by atrial premature contractions. Typically, a localized cause can be found at the junction between the pulmonary veins and the left atrium. In these instances, catheter-based techniques using high-radiofrequency energy, balloon cryoablation, or pulse-field energy may be applied to electrically isolate the pulmonary veins from the left atrium, thereby effectively treating AF (Fig. 2).
- In cases where severe bradycardia is concurrent with AF, such as in tachycardia-bradycardia syndrome, where antiarrhythmic drugs could not be an option, initial pacemaker placement and antiarrhythmic drugs would be an option or an initial intervention may be necessary.
- The recommendation is to carry out this procedure when symptoms such as low blood pressure and congestive heart failure occur due to rapid ventricular contractions. This also applies to cases of tachycardia-induced cardiomyopathy that are solely caused by bradycardia, if medication does not adequately manage the condition.
- During catheter ablation procedures, there is little risk of thromboembolism in patients. Consequently, when using this procedure to achieve rhythm control, all patients, irrespective of their risk level, must undergo anticoagulation therapy for at least 2 months.
RADIOFREQUENCY CATHETER ABLATION
- Electrode catheter ablation is a technique developed for the treatment of arrhythmias. At present, it is recognized as one of the standard treatment approaches for patients suffering from symptomatic AF. Given the intricate pathophysiology involved in the initiation and progression of AF, catheter ablation has been limited to a specific subset of patients. However, with the evolution of catheter ablation technology, particularly the use of three-dimensional mapping systems, and continuous research into the root causes, this procedure is now being performed on a larger number of patients. In some instances, it is even being used as a primary treatment option [3,4].
- Indications
- Radiofrequency catheter ablation (RFCA) may be indicated for AF patients who continue to experience symptoms, despite undergoing rhythm control therapy with antiarrhythmic drugs. The goal of this procedure is to alleviate various symptoms.
CRYOBALLOON ABLATION
- Although RFCA is widely used for the treatment of AF patients, it is known for its procedural complexity, extended duration, and dependence on the operator's skills. As a result, the development of new procedural techniques is underway. Cryoballoon ablation, a procedure that isolates the pulmonary veins using a balloon, has demonstrated a reduction in procedure time and improved safety when compared to traditional RFCA. Furthermore, economic evaluations have indicated that cryoballoon ablation is highly cost-effective [3–5].
- Indications
- Cryoballoon ablation is typically used for paroxysmal or persistent AF patients requiring pulmonary vein isolation. However, for patients with longstanding persistent AF in need of treatment, or those experiencing multiple concurrent arrhythmias (such as atrial flutter, paroxysmal ventricular tachycardia, etc.), the use of RFCA with three-dimensional mapping systems is typically more beneficial.
KOREAN HEALTH INSURANCE COVERAGE CRITERIA FOR AF RFCA/CRYOBALLOON ABLATION
- The coverage criteria for AF RFCA and cryoballoon ablation under Korea's National Health Insurance are as follows.
- (1) AF that persists despite the administration of at least one antiarrhythmic drug (class I or III) at an adequate dose for more than 6 weeks, and AF is confirmed by an electrocardiogram before and after drug therapy.
- (2) In cases where drug therapy has failed or cannot be administered due to adverse effects of antiarrhythmic drugs or in the presence of sick sinus syndrome (tachycardia-bradycardia syndrome) accompanied by AF confirmed by electrocardiography.
- (3) Repeat procedures should be performed at least 3 months after the previous procedure, but only when AF or atrial flutter recurrence is confirmed on the electrocardiogram.
- (4) Atrial flutter RFCA is indicated when cavotricuspid isthmus–dependent atrial flutter is confirmed.
HYBRID TREATMENT USING THORACOSCOPIC ARRHYTHMIA SURGERY: LONGSTANDING PERSISTENT AF TREATMENT
- Thoracoscopic arrhythmia surgery is a procedure that uses thoracoscopy to directly visualize the heart and ablate the areas causing arrhythmias with radiofrequency energy. It also involves blocking the left atrial appendage, which is well-known for causing strokes. The advantages of thoracoscopic arrhythmia surgery include performing the surgery while the heart is beating, reducing the surgical duration compared to traditional procedures that require stopping the heart, increasing stability. The incision is approximately 5 mm long, resulting in minimal scarring, mild discomfort, and a quick recovery after surgery. The success rate of thoracoscopic arrhythmia surgery greatly depends on the proficiency of the surgeon.
- In approximately 30% of patients, residual areas of AF may remain after thoracoscopic surgery. In such cases, a hybrid treatment approach is employed, combining an intracardiac procedure with catheter ablation to remove the areas responsible for arrhythmia both inside and outside the heart. Hybrid AF ablation is a collaborative treatment method that involves minimal invasive thoracoscopic arrhythmia surgery and percutaneous intracardiac RFCA for patients for whom complete recovery is challenging or those who experience recurrent arrhythmias after the procedure [3,4,6].
- Indications
- This approach is advantageous for patients with a history of stroke associated with AF, individuals with a high risk of stroke or recurrence in longstanding persistent AF, and those who experience recurrences following catheter ablation.
CONCLUSIONS
- As the population ages, the number of patients with AF is increasing. The goals of treatment include improving symptoms, restoring ventricular function, preventing thromboembolism, and reducing mortality [1]. Therefore, upon diagnosing AF, it is crucial to first identify and eliminate any underlying causes or triggering factors. If symptoms of AF are present and cardiac function is impaired, active rhythm control becomes necessary. When choosing medications, their effectiveness and potential side effects should be evaluated in accordance with the principles of antiarrhythmic drug use. If drug therapy is ineffective, produces adverse effects, or if drug usage is problematic due to issues such as tachycardia-bradycardia syndrome, alternatives such as RFCA, cryoballoon ablation, or thoracoscopic surgical procedures may be considered. In instances of asymptomatic AF that is incidentally discovered, particularly in elderly patients with longstanding persistent AF who do not exhibit symptoms like heart failure, palpitations, dizziness, or other symptoms, it may be appropriate to administer anticoagulants solely for stroke prevention when the heart rate is normal. This strategy considers the potential side effects of antiarrhythmic medications and the possibility of recurrence following procedures or surgeries. Monitoring the patient's progress is vital, with a focus on comprehensive care for patients with AF.
ARTICLE INFORMATION
-
Conflicts of interest
The author has no conflicts of interest to declare.
-
Funding
The author received no financial support for this study.
Fig. 1.Strategies for antiarrhythmic drug use. LVH, left ventricular hypertrophy; LV, left ventricular; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; MRA, mineralocorticoid-receptor antagonist; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; AFCA, atrial fibrillation catheter ablation; AF, atrial fibrillation.

Fig. 2.Schematic of atrial fibrillation treatment. (A) Cryoballoon catheter ablation. (B) Radiofrequency catheter ablation.
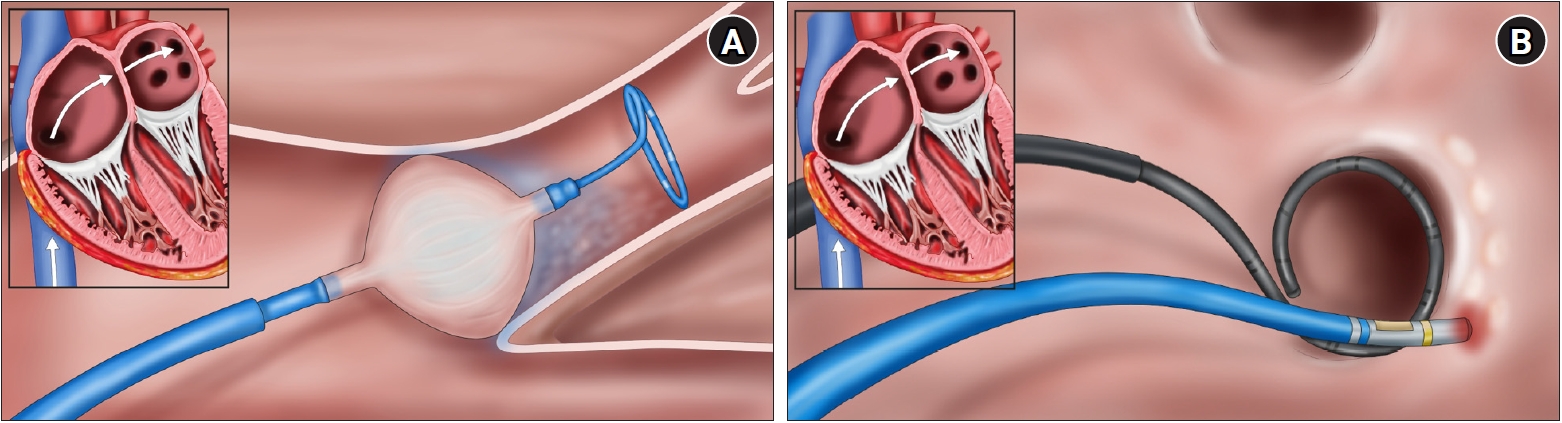
Table 1.Nonvitamin K oral anticoagulants
|
Dabigatran |
Apixaban |
Rivaroxaban |
Edoxaban |
|
Site of action |
Factor II |
Factor X |
Factor X |
Factor X |
|
Dosing |
150 mg twice a day |
5 mg twice a day |
20 mg once a day |
60 mg once a day |
|
Dose reduction |
110 mg twice a day |
2.5 mg twice a day |
15 mg once a day |
30 mg once a day |
|
Dose reduction criteria |
CrCl 30–49 mL/min |
At least two of three: |
CrCl 15–49 mL/min or in addition to antiplatelet therapy |
Weight ≤60 kg |
|
Age ≥80 yr |
1. Weight ≤60 kg |
CrCl 15–49 mL/min |
|
Verapamil |
2. Age ≥80 yr |
Strong P-Gp inhibitor (dronedarone, ciclosporin, erythromycin, ketoconazole) |
|
3. sCr ≥1.5 mg/dL |
|
|
Or CrCl 15–29 mL/min |
|
|
Interaction with amiodarone |
+12%–60% |
No data |
Minor effect |
+40% |
|
Dronedarone |
+70%–100% |
With caution |
Moderate effect, should be avoided |
+85% (dose reduction to 30 mg) |
|
Verapamil |
+12%–180% (reduction to 110 mg twice a day) |
No data |
+40% |
+53% |
|
Clarithromycin, erythromycin |
Clarithromycin +19% AUC |
Clarithromycin +60% AUC |
Clarithromycin +50% AUC |
Erythromycin +85% AUC (dose reduction to 30 mg) |
|
Erythromycin +19% AUC |
|
CrCl |
≥50 mL/min: 150 mg twice a day |
≥50 mL/min: 2.5–5 mg twice a day |
≥50 mL/min: 20 mg once a day |
≥50 mL/min: 60 mg once a day |
|
30–49 mL/min: 110 mg twice a day |
30–49 mL/min: 2.5–5 mg twice a day |
30–49 mL/min: 15 mg once a day |
30–49 mL/min: 30 mg once a day |
|
<30 mL/min: should be avoided |
15–30 mL/min: 2.5 mg twice a day |
15–30 mL/min: 15 mg once a day |
15–30 mL/min: 30 mg once a day |
|
Liver disease |
|
|
|
|
|
Child-Pugh class A |
Normal dose |
Normal dose |
Normal dose |
Normal dose |
|
Child-Pugh class B |
With caution |
With caution |
Not recommended |
With caution |
|
Child-Pugh class C |
Not recommended |
Not recommended |
Not recommended |
Not recommended |
Table 2.Antiarrhythmic drugs
|
Drug |
Indication |
Contraindication |
Dosing |
Common side effect |
|
Flecainide |
AF |
AV block, sick sinus syndrome, prolonged QTc (>500 msec), ischemic heart disease, congestive heart failure |
50–100 mg twice a day |
AFL with 1:1 |
|
QRS complex widening |
|
Propafenone |
AF |
AV block, sick sinus syndrome, prolonged QTc (>500 msec), ischemic heart disease, congestive heart failure |
225–425 mg twice a day |
AFL with 1:1 |
|
QRS complex widening |
|
Sotalol |
AF with ischemic heart disease |
AV block, sick sinus syndrome, prolonged QTc (>500 msec), congestive heart failure |
40–80 mg twice a day |
QT prolongation |
|
Amiodarone |
AF with congestive heart failure, VT |
AV block, sick sinus syndrome, prolonged QTc (>500 msec) |
200 mg once a day |
Hypotension, bradycardia, AV block, QT prolongation, corneal microdeposits, thyroid function test abnormality, pulmonary toxicity |
|
Dronedarone |
AF with ischemic heart disease |
AV block, congestive heart failure (NYHA class III or IV) |
400 mg twice a day |
Bradycardia, AV block, QT prolongation, abnormal liver function |
REFERENCES
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498.ArticlePubMed
- 2. Yamanouchi H, Nagura H, Mizutani T, Matsushita S, Esaki Y. Embolic brain infarction in nonrheumatic atrial fibrillation: a clinicopathologic study in the elderly. Neurology 1997;48:1593–7.ArticlePubMed
- 3. Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J 2017;38:20–6.ArticlePubMedPMC
- 4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160.Article
- 5. Chen S, Purerfellner H, Ouyang F, Kiuchi MG, Meyer C, Martinek M, et al. Catheter ablation vs. antiarrhythmic drugs as ‘first-line’ initial therapy for atrial fibrillation: a pooled analysis of randomized data. Europace 2021;23:1950–60.ArticlePubMedPDF
- 6. On YK, Jeong DS. Updates in hybrid AF ablation: a hybrid approach to surgical epicardial ablation and cather endocardial ablation in persistent atrial fibrillation. Int J Arrhythm 2022;23:5. ArticlePDF
Citations
Citations to this article as recorded by




