ABSTRACT
-
Background
- The goal of the study was to investigate changes in cardiac function during iron chelating therapy (ICT) in patients with transfusion-induced iron overload.
-
Methods
- We prospectively examined cardiac function in 21 aplastic anemia patients for 2 years by using transthoracic echocardiography before and during ICT.
-
Results
- The serum ferritin level decreased from 4,961.5±2,917.9 µg/L to 2,466.9±2,533.1 µg/L after 2 years (P<0.001). The left ventricular ejection fraction decreased to under the normal limit (55%) in five patients. The serum ferritin level was positively correlated with the E/E’ ratio (r=0.595, P=0.004) and the left atrial (LA) volume (r=0.685, P=0.001) and negatively correlated with the deceleration time (r=–0.586, P=0.005) after 2 years of ICT. The seven responders (serum ferritin level <1,000 µg/L after 2 years of ICT) demonstrated a significantly higher ejection fraction, smaller LA volume and left ventricular end-systolic dimension, and a slower deceleration time than the 14 nonresponders (≥1,000 µg/L).
-
Conclusions
- These results suggest that the response to ICT, which was estimated by the serum ferritin level, can reflect cardiac function during ICT. In nonresponders, cardiac function monitoring during ICT may be helpful for the early detection of cardiac dysfunction.
-
Keywords: Aplastic anemia; Iron overload; Deferasorox; Echocardiography
INTRODUCTION
- Aplastic anemia is characterized by peripheral cytopenia and a decrease in bone marrow cellularity, necessitating multiple transfusions of packed red blood cells (PRCs) throughout the disease's progression [1]. The repeated PRC transfusions can lead to elevated serum iron levels, which can inflict damage on various organs [2]. Iron accumulation in cardiac myocytes can trigger fatal arrhythmias and cardiomyopathy, making cardiac complications a leading cause of death in transfusion-dependent hematological conditions [3,4]. Magnetic resonance imaging is the most effective tool for assessing cardiac function and the extent of iron accumulation in the myocardium [4–7]. Numerous studies have utilized transthoracic echocardiography (TTE) to examine patients with transfusion-induced iron overload (TIO) [8–13]. TTE assessments have shown that diastolic left ventricular (LV) dysfunction can precede systolic LV dysfunction, even in patients who do not exhibit heart failure symptoms [8,9,12].
- Iron chelating therapy (ICT) is known to be effective in reducing the iron burden in patients with TIO, and it can also aid in the recovery of damaged organ functions [14–16]. It is not uncommon to observe the recovery of cardiac function from severe symptomatic heart failure following ICT, as documented in several case reports [17–20]. Deferasirox (Exjade, Novartis) is an oral iron chelating agent that offers a longer half-life and fewer side effects than traditional iron chelating agents [21,22]. It has also demonstrated excellent chelating efficiency in myelodysplastic syndromes, including aplastic anemia [23–25]. While there are guidelines for managing TIO [26], they do not specify whether cardiac function should be evaluated during the disease's progression. For patients with aplastic anemia who show no symptoms or signs of heart failure, cardiac function is not typically evaluated during ICT. In this study, we used TTE to examine changes in cardiac function during ICT with deferasirox in patients with aplastic anemia who were asymptomatic for heart failure. The aim of this study was to ascertain the usefulness of monitoring cardiac function during ICT and to observe the changes in cardiac function during ICT in patients with TIO.
METHODS
- Ethics statements
- This study was approved by the Institutional Review Board of Yeouido St. Mary’s Hospital (No. SC10RISI0047). The requirement for informed consent was waived due to the retrospective nature of the study.
- Subjects
- In this study, we examined cardiac function in 25 consecutive patients diagnosed with aplastic anemia and TIO using TTE at Yeouido St. Mary’s Hospital, The Catholic University of Korea (Seoul, Korea). Each patient's diagnosis of aplastic anemia was confirmed through a bone marrow biopsy. We excluded any patients with cardiac risk factors such as a history of coronary artery disease, hypertension, or diabetes mellitus. TIO was identified as a transferrin saturation exceeding 50% and a serum ferritin level surpassing 350 g/L [27]. Patients who developed TIO received treatment with an oral chelating agent, deferasirox (Exjade), following the guidelines proposed by a Japanese hematology group [26]. ICT was initiated when the serum ferritin level exceeded 1,000 g/L. The dosage of deferasirox was adjusted in accordance with serum ferritin levels, which were regularly monitored. ICT was continued until the serum ferritin level dropped below 500 g/L. There were no restrictions on the treatment of aplastic anemia. Patients who demonstrated poor compliance or discontinued medication due to adverse effects were not included in this analysis.
- Cardiac function evaluation by TTE
- Cardiac function was examined using TTE (Sonos 5500, Hewlett-Packard) prior to deferasirox administration. We measured M-mode, two-dimensional, and Doppler echocardiographic parameters for LV function. We monitored changes in cardiac function at 6, 12, and 24 months of ICT using TTE (Fig. 1). A single echocardiographer conducted all TTE examinations, which were video recorded. Two experts from Yeouido St. Mary’s Hospital reviewed and interpreted these examinations. We measured chamber size and wall thickness, and evaluated parameters of LV systolic and diastolic function. LV systolic dysfunction was defined as an LV ejection fraction (LVEF) that was more than 10% points below baseline or an LVEF below 55%. Diastolic function was evaluated by measuring peak early mitral inflow velocity (E), peak atrial systolic mitral inflow velocity (A), and deceleration time (DT) using Doppler echocardiography. We also measured peak early diastolic annular velocity (E’) and peak atrial systolic annular velocity (A’) using tissue Doppler imaging of the septal annulus. The following four patterns of diastolic function were defined as follows: (1) normal LV filling, where E>A and E’>A’; (2) abnormal LV relaxation, where E<A and E’<A’; (3) pseudonormal LV filling, where E>A and E’<A’; and (4) restrictive LV filling, where E>>A and E’<A’. The E/E’ ratio was used to predict the LV filling pressure in cases of abnormal LV filling patterns.
- Statistical analysis
- Continuous variables are presented as mean±standard deviation, and categorical data are presented as absolute values and percentages. Statistical analysis was performed using nonparametric tests. The parameters before and during ICT were compared using the Wilcoxon signed-rank test. Statistical significance was set at P<0.05. Correlations between serum ferritin concentrations and echocardiographic parameters were analyzed using Spearman coefficients. Statistical analysis was performed using SAS ver. 9.1 (SAS Institute).
RESULTS
- Clinical characteristics
- Twenty-one out of 25 patients completed 2 years of follow-up; one patient died secondarily due to a severe infection and two patients stopped deferasirox due to gastrointestinal side effects. One patient refused a follow-up TTE at the 12-month time point and was excluded from the analysis. The mean patient age was 31±8 years and the male to female ratio was 12:9. The clinical characteristics of patients are summarized in Table 1. One patient had a first-degree atrioventricular (AV) block and three patients had LV hypertrophy (LVH) determined by voltage criteria on an electrocardiogram (ECG) at the time of enrollment. No patient presented with symptoms or signs of congestive heart failure. Four of the 21 patients were treated with bone marrow transplantation during the follow-up period and deferasirox administration was maintained until the serum ferritin level fell below 500 μg/L. Eleven patients were treated with immunosuppressive agents, including cyclosporine with antithymocyte globulin or antilymphocyte globulin. Six patients underwent conservative treatment with repeated transfusions. After 2 years of treatment with deferasirox, the mean serum ferritin level significantly decreased from 4,961.5±2,917.9 to 2,466.9±2,533.1 µg/L (P<0.001). Only seven patients reached a serum ferritin level under 1,000 μg/L, which is the recommended concentration of serum ferritin in patients with secondary iron overload [26], and these patients were classified as responders. Two patients, who were treated with bone marrow transplantation (patient 19) and immunosuppressive therapy (patient 15), reached a serum ferritin level under 500 μg/L and discontinued deferasirox treatment. Four patients (patients 1, 7, 9, and 14), who had a longer disease duration and a greater amount of transfused PRCs, demonstrated an increased serum ferritin level at the end of follow-up.
- After 2 years, there were no interval changes in the patients with first-degree AV block and LVH by voltage criteria on ECG; however, one patient developed atrial fibrillation. None of the patients developed symptoms or signs of heart failure after 2 years.
- Echocardiographic parameters
- A comparison of initial echocardiographic parameters and follow-up studies during ICT demonstrated no significant differences, except for interventricular septum thickness (IVS) and the right ventricular (RV) wall thickness (Table 2). After undergoing ICT for 2 years, five patients (patients 1, 2, 4, 13, and 17) (Table 3) demonstrated LV systolic dysfunction (LVEF, <55%), but three of these (patients 1, 2, and 4) had LV systolic dysfunction prior to initiating ICT. The LVEF of two patients (patients 13 and 17), who had an LVEF ≥55% prior to ICT, decreased to <55% during ICT. Three out of the four patients (patients 7, 9, and 14) who demonstrated an increased serum ferritin level during ICT, maintained a LVEF above 55%. Four patients (patients 1, 2, 7, and 20) demonstrated abnormal diastolic function patterns prior to ICT. One patient (patient 2) had an abnormal relaxation pattern and three patients (patients 1, 7, and 20) had a pseudonormal relaxation pattern. All four patients with abnormal diastolic function patterns demonstrated a relatively large LA volume and were included in the nonresponder group after 2 years of ICT. The E/E’ ratio of patient 2 increased after treatment, which suggested an increased LV filling pressure after treatment. As suggested by changes in Doppler findings, patients 7 and 20 may have attained improved diastolic function, from a pseudonormal to a normal relaxation pattern, and with no significant increase in the E/E’ ratio. Patient 1, who demonstrated a decreased LVEF during ICT, developed a restrictive physiology pattern and was the only patient who demonstrated progression in LV systolic and diastolic dysfunction, according to the echocardiographic parameters, and also developed atrial fibrillation during ICT. This particular patient did not respond to ICT at all; the serum ferritin level was as high as 10,931 μg/L, even while maintaining a high dose of deferasirox, which may have been the reason why the cardiac dysfunction was aggravated. Two patients (patients 1 and 2) demonstrated simultaneous systolic and diastolic dysfunction. There was only one patient (patient 18) who progressed to an abnormal relaxation pattern after 2 years of ICT from an initial normal relaxation. This patient was classified as a responder, and the E/E’ ratio and LA volume were within normal limits, suggesting normal LV filling pressure.
- Comparison of responder and nonresponder groups
- At the time of enrollment, there were no significant differences between the two groups (Table 4). After 2 years of ICT, the serum ferritin levels were significantly lower in the responder group than the nonresponder group (514.14±246.76 μg/L vs. 2,740.28±1,531.13 μg/L, P<0.001). Regarding the echocardiographic parameters, there were significant differences in LV end-systolic dimension (LVEsD), LA volume, LVEF, and DT. Although the LVEsD of the responder group was smaller than that of the nonresponder group (29.11±4.2 mm vs. 34.09±5.3 mm, P=0.038) and the LVEF of the responder group was higher than the nonresponder group (61.18%±3.80% vs. 56.70%±4.86%, P=0.038) after 2 years of ICT, both parameters remained within normal limits in the nonresponder group. This may suggest that there were no clinically meaningful differences between the two groups. The LA volume of the nonresponder group was larger than the responder group (45.71±11.14 mL vs. 63.43±16.32 mL, P=0.012) and the age-related normal limit.
- Correlation of serum ferritin levels and echocardiographic parameters
- Prior to initiating ICT, no echocardiographic parameters demonstrated correlations with the concentration of serum ferritin (Fig. 2). After 2 years of ICT, the parameters associated with diastolic function, such as the E/E’ ratio (r=0.595, P=0.004), the DT (r=–0.586, P=0.005), and the LA volume (r=0.658, P=0.001), demonstrated correlations with the concentration of serum ferritin. The parameters that represented systolic function did not demonstrate correlations with serum ferritin levels before or after treatment. These results suggest that higher serum ferritin levels during ICT correspond to higher LV filling pressures.
DISCUSSION
- The serum ferritin level is the most important laboratory test that reflects the body’s iron load [2,28]. Heart failure is a major cause of death for patients with secondary hemochromatosis due to TIO [3,4], and elevated serum ferritin levels are a risk factor for iron deposition in cardiac myocytes [29]. ICT is an effective method for reducing the body's iron load [15] and for improving cardiac function in patients with secondary cardiac hemochromatosis [17,18]. Keeping the serum ferritin level below 2,500 g/L is linked to better survival rates without cardiac disease [16,30]. However, a previous single cross-sectional study found no correlation between ferritin levels and echocardiographic parameters [11], suggesting that a single serum ferritin level measurement may not accurately predict cardiac dysfunction. In our study, we found that ICT with deferasirox was highly effective in reducing serum ferritin levels. However, achieving the target ferritin level was challenging for patients who continuously required PRC transfusions, making deferasirox essential for these patients. While changes in serum ferritin levels during ICT can indicate shifts in the body's iron load [14,16,31], it remains unclear whether changes in serum ferritin levels during and after ICT correlate with changes in cardiac function.
- It has been reported that deferasirox treatment can potentially restore cardiac function in patients with aplastic anemia suffering from significant systolic dysfunction due to TIO [20]. In this study, however, echocardiography parameters did not show significant differences after 2 years of ICT. This lack of significant change in cardiac function may be attributed to the fact that the patients' initial cardiac function was relatively well-preserved, and most patients maintained normal systolic and diastolic LV function after 2 years. While ICT may not have improved the patients' cardiac function, it may have contributed to maintaining cardiac function within normal parameters during the treatment period. Three patients (patients 1, 2, and 4), who initially had a LVEF lower than 55%, did not show improvement after 2 years of ICT, but neither did they exhibit significantly worse LV function. This suggests that even high-risk patients with existing LV systolic dysfunction can be safely treated with deferasirox, potentially preventing further cardiac dysfunction through effective ICT. Two patients who experienced a decline in LVEF from the normal range to below 55% were in the nonresponder group. There were several patients with increased LVEF in the responder group and decreased LVEF in the nonresponder group, but most of these patients did not show significant changes in LVEF. One patient (patient 1), who did not respond to ICT, showed a decrease in LVEF, progression of diastolic dysfunction, and the onset of atrial fibrillation. Even though the statistical analysis of this study did not show a significant difference, it is evident that effective ICT can help preserve cardiac function in cases of TIO.
- A comparison between the responder and nonresponder groups demonstrated that effective ICT can modify cardiac function in TIO patients. Some parameters showed significant differences between the two groups, particularly those related to diastolic function. In a correlation analysis, serum ferritin levels after 2 years of ICT were found to correlate solely with echocardiographic parameters of diastolic function, but not with systolic function parameters. Diastolic parameters during ICT, such as the E/E’ ratio, LA volume, and DT, were found to correlate with serum ferritin levels. These findings suggest that an increase in LV filling pressure corresponds to a higher serum ferritin level. Sixteen patients who initially had normal relaxation patterns prior to ICT continued to maintain these patterns during the therapy. One patient's relaxation pattern progressed from normal to abnormal, but the LV filling pressure was assumed to be normal. Given previous studies suggesting that improvements in diastolic dysfunction precede those in systolic dysfunction [8,9], these results indicate that effective ICT may prevent further cardiac dysfunction in patients with only diastolic dysfunction.
- This study also demonstrated that effective ICT results in changes in cardiac structures. The reduced IVS thickness and RV wall thickness after 2 years of ICT may reflect decreased concentrations of iron within the cardiac myocytes, based on our previous observations that a group of patients with aplastic anemia had greater RV wall thickness than normal controls [10]. This suggests that effective ICT can also prevent the deterioration of cardiac structures.
- Numerous studies have examined the impact of TIO on cardiac function in patients with thalassemia major, but there are fewer comparable studies involving patients with aplastic anemia. The effects of TIO may vary between the clinical progression of thalassemia major and aplastic anemia patients, and the cardiac function of aplastic anemia patients receiving TIO has not been as extensively researched as in patients with thalassemia major receiving TIO. The influence of ICT on cardiac function in patients already experiencing cardiac dysfunction has been the focus of many previous reports, but similar studies are lacking in patients receiving TIO who have normal cardiac function. To the best of our knowledge, this is the inaugural study that tracked cardiac function in patients with TIO before and during ICT over a 2-year period and compared the changes in cardiac function between responders and nonresponders to ICT in aplastic anemia patients. Regular monitoring of serum ferritin levels is necessary during ICT to assess treatment response, as the response to ICT can predict changes in cardiac function in TIO patients. It is evident that a positive response to ICT can maintain cardiac function, while a poor response to ICT may indicate cardiac dysfunction, particularly diastolic function, and a decline in cardiac structures. It is plausible that chronic nonresponders may eventually develop systolic LV dysfunction. We propose that patients who respond well to ICT may not require cardiac function monitoring, but nonresponders, or patients who fail to achieve the therapeutic target of ICT, may need cardiac function monitoring during their clinical management.
- There are some limitations to the present study. First, the enrolled patients were a heterogeneous population with various disease durations and different numbers of PRC transfusions at baseline. This could have led to varying levels of iron accumulation prior to ICT, which might explain the inconsistent response to ICT. Second, the therapeutic approaches used to treat aplastic anemia varied among patients. The cardiotoxic properties of certain treatment drugs, such as cyclosporin, could have potentially impacted the patients' cardiac function. Third, this was an observational study with a single group and a relatively small sample size. If we had compared the cardiac effects of ICT with those of an untreated group, we might have gained more insight into the impact of ICT on cardiac function. However, securing a control group for this study population is challenging due to ethical concerns about not treating TIO in these patients. Finally, all patients had good cardiac function prior to ICT, making it nearly impossible to demonstrate any improvement in cardiac function after 2 years of ICT. Despite these limitations, this study clearly showed that cardiac function remained within normal parameters in patients who responded to ICT.
- To summarize, we conducted a prospective 2-year follow-up study using TTE to investigate the impact of ICT on the cardiac function of patients with aplastic anemia. Our findings indicated that ICT, specifically with deferasirox, triggers alterations in cardiac structure and function. We identified a correlation between the concentration of serum ferritin and the diastolic echocardiographic parameters after 2 years of ICT. However, no such relationship was observed prior to the treatment. It is essential to regularly measure the serum ferritin level to monitor the patient's response to ICT. The cardiac function of patients with aplastic anemia is not typically assessed during the disease progression. However, those who do not respond to ICT necessitate cardiac function monitoring, even in the absence of heart failure symptoms or signs. The optimal timing for cardiac function monitoring in nonresponders to ICT remains unclear, necessitating further research. This research should aim to clarify not only the ideal timing for cardiac function monitoring but also the most effective management strategies to minimize cardiac injury from TIO.
ARTICLE INFORMATION
-
Ethics statements
This study was approved by the Institutional Review Board of Yeouido St. Mary’s Hospital (No. SC10RISI0047). The requirement for informed consent was waived due to the retrospective nature of the study.
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
This work was supported by the Catholic Research Institute for Intractable Cardiovascular Disease (CRID), College of Medicine, The Catholic University of Korea (Seoul, Korea).
Fig. 1.Cardiac function and response monitoring during iron chelating therapy (ICT). Serum ferritin concentrations were measured regularly every 2 months, and transthoracic echocardiography (TTE) was conducted before ICT and at 6, 12, and 24 months of ICT.
Fig. 2.Relationship between serum ferritin levels and echocardiographic parameters of diastolic function. Left atrial (LA) volume, peak early mitral inflow velocity (E) to peak early diastolic annular velocity (E’) ratio, and deceleration time (DT) did not demonstrate a relationship with serum ferritin levels before iron chelating therapy with deferasirox. The LA volume and E/E’ ratio demonstrated positive correlations with serum ferritin levels, and the DT demonstrated negative correlations with ferritin levels after treatment. (A) Pretreatment and (B) posttreatment LA volume. (C) Pretreatment and (D) posttreatment E/E’ ratio. (E) Pretreatment and (F) posttreatment DT.
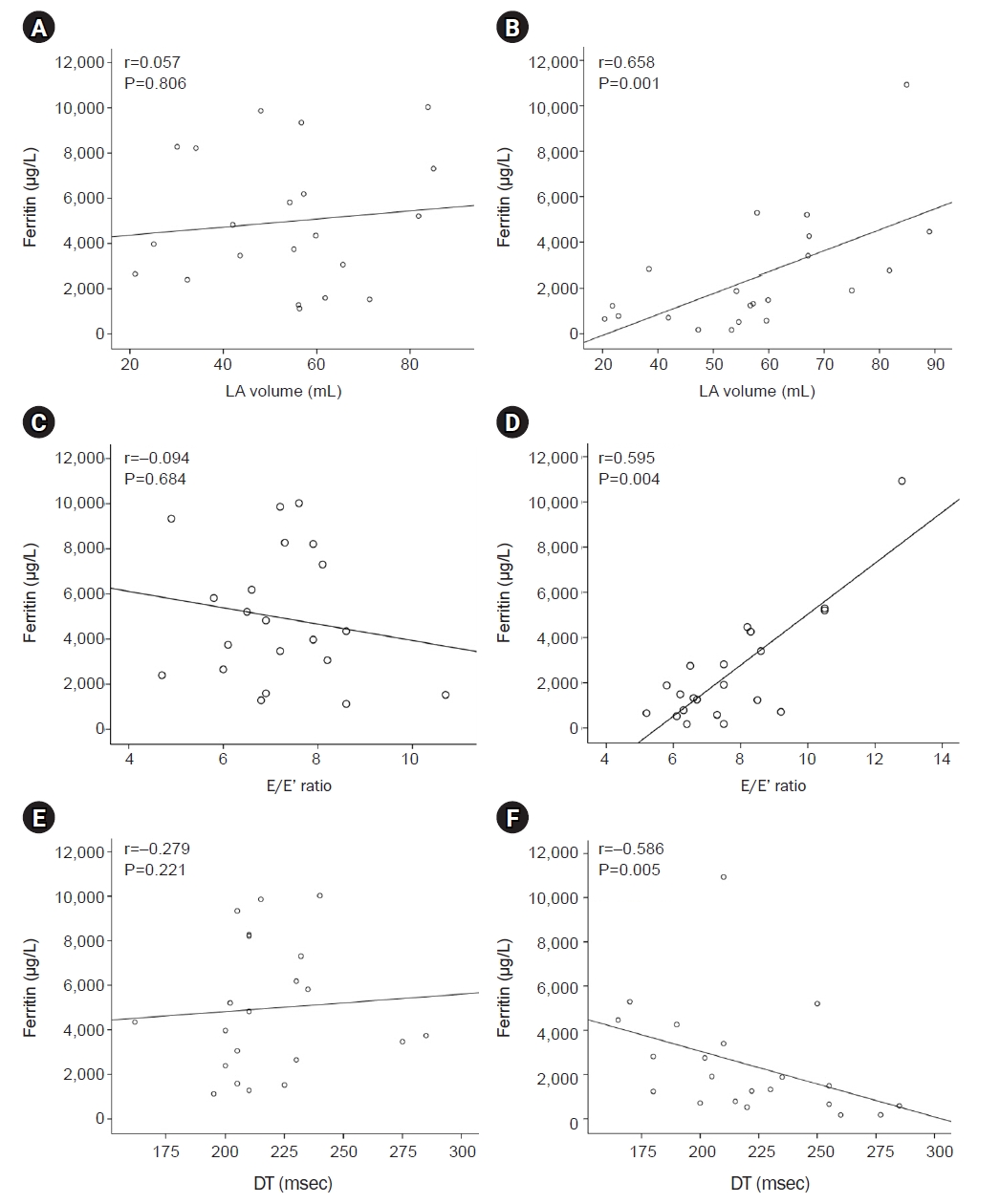
Table 1.Clinical characteristics of patients
|
Patient no. |
Age (yr) |
Sex |
Hemoglobin (mg/dL) |
Transfusion amount (U) during ICT |
Period with disease (mo) |
Serum ferritin (μg/L) |
Maintenance dose of deferasirox (mg) |
Electrocardiogram |
Treatment |
|
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
|
1 |
32 |
Male |
6.6 |
68 |
102 |
10,026 |
10,931 |
2,125 |
NSR |
Atrial fibrillation |
CsA |
|
2 |
39 |
Male |
6.2 |
136 |
78 |
7,307 |
5,202 |
1,750 |
NSR |
No change |
Conservative |
|
3 |
26 |
Female |
7.6 |
16 |
46 |
2,393 |
523 |
600 |
NSR |
No change |
CsA+ALG |
|
4 |
23 |
Male |
7.1 |
56 |
34 |
6,184 |
1,326 |
625 |
First-degree AV block |
No change |
Conservative |
|
5 |
26 |
Female |
9.0 |
76 |
70 |
9,863 |
2,813 |
875 |
LVH by voltage |
No change |
Conservative |
|
6 |
20 |
Female |
5.3 |
147 |
64 |
4,823 |
4,261 |
1,000 |
NSR |
No change |
Conservative |
|
7 |
49 |
Male |
6.6 |
80 |
83 |
3,059 |
3,399 |
2,125 |
NSR |
No change |
CsA+ALG |
|
8 |
28 |
Female |
7.5 |
23 |
103 |
4,345 |
783 |
625 |
LVH by voltage |
No change |
BMT |
|
9 |
45 |
Male |
12.5 |
38 |
69 |
1,589 |
1,912 |
625 |
NSR |
No change |
BMT |
|
10 |
37 |
Female |
7.7 |
48 |
41 |
3,971 |
710 |
625 |
NSR |
No change |
CsA+ALG |
|
11 |
45 |
Female |
6.9 |
70 |
77 |
8,212 |
5,293 |
1,000 |
NSR |
No change |
Conservative |
|
12 |
25 |
Female |
5.2 |
100 |
87 |
1,524 |
1,487 |
1,000 |
NSR |
No change |
CsA+ALG |
|
13 |
21 |
Male |
7.0 |
118 |
128 |
9,339 |
4,460 |
1,500 |
NSR |
No change |
Conservative |
|
14 |
28 |
Female |
8.5 |
16 |
202 |
1,124 |
1,253 |
1,000 |
NSR |
No change |
CsA+ALG |
|
15 |
32 |
Male |
13.9 |
21 |
143 |
3,464 |
172 |
- |
NSR |
No change |
ALG |
|
16 |
29 |
Female |
6.9 |
103 |
87 |
5,816 |
1,884 |
1,125 |
NSR |
No change |
CsA+ATG |
|
17 |
27 |
Male |
7.4 |
41 |
58 |
8,272 |
1,234 |
1,000 |
NSR |
No change |
CsA+ATG |
|
18 |
33 |
Male |
14.9 |
13 |
43 |
2,650 |
654 |
600 |
NSR |
No change |
BMT |
|
19 |
30 |
Male |
6.5 |
2 |
32 |
1,280 |
177 |
- |
LVH by voltage |
No change |
BMT |
|
20 |
31 |
Male |
5.2 |
12 |
156 |
5,208 |
2,749 |
750 |
NSR |
No change |
ALG, ATG |
|
21 |
40 |
Male |
12.2 |
7 |
30 |
3,742 |
580 |
600 |
NSR |
No change |
CsA+ALG |
Table 2.Comparison of echocardiographic parameters
|
Echocardiographic parameter |
Initial |
2 yr Follow-up |
P-value |
|
IVS thickness (mm) |
9.6±1.3 |
8.5±1.6 |
0.007 |
|
LVPW thickness (mm) |
10.2±1.7 |
9.8±2.0 |
0.250 |
|
RV wall thickness (mm) |
3.5±0.5 |
2.8±.0.6 |
0.001 |
|
LV end-diastolic dimension (mm) |
49.5±4.5 |
49.8±6.5 |
0.150 |
|
LV end-systolic dimension (mm) |
31.8±3.8 |
32.4±5.4 |
0.720 |
|
LA dimension (mm) |
36.9±5.4 |
37.8±6.4 |
0.690 |
|
LA volume (mL) |
46.2±16.5 |
61.3±17.8 |
0.090 |
|
LV mass index (mg/m2) |
81.5±11.8 |
81.8±14.9 |
0.910 |
|
Fractional shortening (%) |
35.7±3.8 |
37.1±4.9 |
0.200 |
|
LV ejection fraction (%) |
57.5±3.9 |
58.2±4.9 |
0.480 |
|
E/E’ |
7.2±1.3 |
7.7±1.8 |
0.220 |
|
Deceleration time (msec) |
218.1±26.8 |
219.8±34.3 |
0.790 |
Table 3.Changes in left ventricular function after 2 years
|
Patient no. |
ICT responder |
LVEF (%) |
E/A ratio |
E’/A’ ratio |
Pattern of diastolic function |
E/E’ ratio |
LA volume (mL) |
|
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
Initial |
2 yr Follow-up |
|
1 |
No |
51 |
46.1 |
2.0 |
2.4 |
0.8 |
0.8 |
P |
R |
7.6 |
15.8 |
83.8 |
84.9 |
|
2 |
No |
53.0 |
52.7 |
0.9 |
0.8 |
0.8 |
0.6 |
A |
A |
8.1 |
10.5 |
85.0 |
66.9 |
|
3 |
Yes |
59.8 |
63.1 |
1.6 |
1.7 |
1.4 |
1.4 |
N |
N |
4.7 |
6.1 |
32.3 |
54.6 |
|
4 |
No |
52.6 |
52.6 |
1.7 |
1.7 |
1.0 |
1.3 |
N |
N |
6.6 |
6.6 |
57.2 |
57.2 |
|
5 |
No |
56.3 |
63.3 |
1.4 |
1.5 |
1.3 |
1.5 |
N |
N |
7.2 |
7.5 |
48.0 |
38.4 |
|
6 |
No |
57.7 |
63.5 |
2.4 |
2.0 |
1.0 |
2.0 |
N |
N |
6.9 |
8.3 |
42.0 |
67.3 |
|
7 |
No |
56.6 |
61.7 |
1.2 |
0.9 |
0.8 |
0.8 |
P |
A |
8.2 |
8.6 |
65.6 |
67.1 |
|
8 |
Yes |
55.4 |
64.9 |
1.8 |
1.6 |
1.8 |
1.6 |
N |
N |
8.6 |
6.3 |
59.8 |
32.9 |
|
9 |
No |
57.7 |
55.7 |
1.3 |
2.1 |
1.3 |
1.3 |
N |
N |
6.9 |
7.5 |
61.8 |
75.0 |
|
10 |
Yes |
55.0 |
56.7 |
1.6 |
1.6 |
1.8 |
1.2 |
N |
N |
7.9 |
9.2 |
25.1 |
41.9 |
|
11 |
No |
60.6 |
59.8 |
1.2 |
1.5 |
1.3 |
1.4 |
N |
N |
7.9 |
10.5 |
34.1 |
57.9 |
|
12 |
No |
58.8 |
58.6 |
1.7 |
1.8 |
1.9 |
1.8 |
N |
N |
10.7 |
6.2 |
71.3 |
59.9 |
|
13 |
No |
57.5 |
53.6 |
1.8 |
1.7 |
1.2 |
1.8 |
N |
N |
4.9 |
8.2 |
56.7 |
89.0 |
|
14 |
No |
69.8 |
60.0 |
1.4 |
1.6 |
1.9 |
2.2 |
N |
N |
8.6 |
6.7 |
56.3 |
56.7 |
|
15 |
Yes |
61.8 |
64.8 |
1.7 |
1.3 |
1.0 |
1.0 |
N |
N |
7.2 |
6.4 |
43.6 |
53.3 |
|
16 |
No |
57.5 |
57.5 |
1.5 |
1.5 |
2.0 |
1.8 |
N |
N |
5.8 |
5.8 |
54.2 |
54.2 |
|
17 |
No |
55.4 |
53.4 |
2.1 |
1.7 |
1.4 |
1.4 |
N |
N |
7.3 |
8.5 |
30.1 |
31.8 |
|
18 |
Yes |
55.1 |
63.6 |
1.2 |
0.7 |
1.1 |
1.0 |
N |
A |
6.0 |
5.2 |
21.1 |
30.4 |
|
19 |
Yes |
59.1 |
56.1 |
1.9 |
1.4 |
1.1 |
1.0 |
N |
N |
6.8 |
7.5 |
56.1 |
47.3 |
|
20 |
No |
55.3 |
55.3 |
1.3 |
1.3 |
0.6 |
0.7 |
P |
P |
6.5 |
6.5 |
81.8 |
81.8 |
|
21 |
Yes |
60.7 |
59.1 |
1.0 |
1.6 |
1.1 |
1.5 |
N |
N |
6.1 |
7.3 |
55.1 |
59.6 |
Table 4.Comparison of responder and nonresponder groups
|
Variable |
Initial |
2 yr Follow-up |
|
Responder (n=7) |
Nonresponder (n=14) |
P-value |
Responder (n=7) |
Nonresponder (n=14) |
P-value |
|
Ferritin level (µg/L) |
3,120.72±1,069.69 |
5,881.88±3,135.97 |
0.056 |
514.14±246.76 |
2,740.28±1,531.13 |
<0.001 |
|
IVS thickness (mm) |
8.94±0.79 |
9.91±1.36 |
0.128 |
8.28±0.99 |
8.63±1.80 |
0.535 |
|
LVPW thickness (mm) |
10.28±0.51 |
10.15±2.07 |
0.799 |
10.07±1.29 |
9.07±2.24 |
0.689 |
|
RV thickness (mm) |
3.31±0.57 |
3.52±0.48 |
0.443 |
2.80±0.59 |
2.87±0.64 |
0.799 |
|
LV end-diastolic dimension (mm) |
48.65±3.77 |
49.97±4.87 |
0.535 |
47.72±5.59 |
50.83±6.90 |
0.172 |
|
LV end-systolic dimension (mm) |
31.25±2.32 |
32.20±4.33 |
0.856 |
29.11±4.2 |
34.09±5.3 |
0.038 |
|
LA dimension (mm) |
34.15±4.30 |
38.39±5.51 |
0.110 |
34.7±4.59 |
39.28±6.81 |
0.149 |
|
LA volume (mL) |
41.87±15.84 |
59.13±17.35 |
0.056 |
45.71±11.14 |
63.43±16.32 |
0.012 |
|
LV mass index (g/m2) |
77.37±9.37 |
83.62±12.56 |
0.322 |
75.45±9.56 |
84.94±16.45 |
0.255 |
|
Fractional shortening (%) |
35.64±3.31 |
35.70±4.08 |
0.636 |
39.15±4.82 |
36.11±4.86 |
0.110 |
|
LV ejection fraction (%) |
58.12±2.89 |
57.12±4.46 |
0.400 |
61.18±3.80 |
56.70±4.86 |
0.038 |
|
E/E’ |
6.75±1.29 |
7.3±1.37 |
0.360 |
6.85±1.28 |
8.15±1.96 |
0.110 |
|
Deceleration time (msec) |
223.14±43.86 |
215.64±14.08 |
0.913 |
244.57±32.90 |
207.42±28.57 |
0.025 |
REFERENCES
- 1. Bacigalupo A. Aplastic anemia: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program 2007;2007:23–8.ArticlePubMedPDF
- 2. Beutler EG, Hoffbrand AV, Cook JD. Iron deficiency and overload. Hematology Am Soc Hematol Educ Program 2003;40–61.ArticlePubMedPDF
- 3. Zurlo MG, De Stefano P, Borgna-Pignatti C, Di Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassaemia major. Lancet 1989;2:27–30.ArticlePubMed
- 4. Aessopos A, Berdoukas V, Tsironi M. The heart in transfusion dependent homozygous thalassaemia today: prediction, prevention and management. Eur J Haematol 2008;80:93–106.ArticlePubMedPMC
- 5. Mavrogeni SI, Markussis V, Kaklamanis L, Tsiapras D, Paraskevaidis I, Karavolias G, et al. A comparison of magnetic resonance imaging and cardiac biopsy in the evaluation of heart iron overload in patients with beta-thalassemia major. Eur J Haematol 2005;75:241–7.ArticlePubMed
- 6. Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009;120:1961–8.ArticlePubMedPMC
- 7. Sakuta J, Ito Y, Kimura Y, Park J, Tokuuye K, Ohyashiki K. Estimation of cardiac left ventricular ejection fraction in transfusional cardiac iron overload by R2* magnetic resonance. Int J Hematol 2010;92:708–12.ArticlePubMedPDF
- 8. Spirito P, Lupi G, Melevendi C, Vecchio C. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation 1990;82:88–94.ArticlePubMed
- 9. Kremastinos DT, Tsiapras DP, Tsetsos GA, Rentoukas EI, Vretou HP, Toutouzas PK. Left ventricular diastolic Doppler characteristics in beta-thalassemia major. Circulation 1993;88:1127–35.ArticlePubMed
- 10. Chung WB, Hong EJ, Youn HJ, Park CS, Oh YS, Chung WS, et al. Echocardiographic characteristics related to chronic iron overload in patient with aplastic anemia. Korean Circ J 2006;36:465–71.Article
- 11. Isma’eel H, Chafic AH, El Rassi F, Inati A, Koussa S, Daher R, et al. Relation between iron-overload indices, cardiac echo-Doppler, and biochemical markers in thalassemia intermedia. Am J Cardiol 2008;102:363–7.ArticlePubMed
- 12. Parale GP, Pawar SS, Tapare VS. Assessment of LV diastolic function in patients with beta-thalassemia major with special reference to E/Eann ratio. J Pediatr Hematol Oncol 2009;31:69–73.ArticlePubMed
- 13. Garceau P, Nguyen ET, Carasso S, Ross H, Pendergrast J, Moravsky G, et al. Quantification of myocardial iron deposition by two-dimensional speckle tracking in patients with β-thalassaemia major and Blackfan-Diamond anaemia. Heart 2011;97:388–93.ArticlePubMed
- 14. Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. N Engl J Med 1998;339:417–23.ArticlePubMed
- 15. Maggio A, D’Amico G, Morabito A, Capra M, Ciaccio C, Cianciulli P, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells Mol Dis 2002;28:196–208.ArticlePubMed
- 16. Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood 2003;102:17–24.ArticlePubMedPDF
- 17. Blank R, Wolber T, Maeder M, Rickli H. Reversible cardiomyopathy in a patient with juvenile hemochromatosis. Int J Cardiol 2006;111:161–2.ArticlePubMed
- 18. Fabio G, Minonzio F, Delbini P, Bianchi A, Cappellini MD. Reversal of cardiac complications by deferiprone and deferoxamine combination therapy in a patient affected by a severe type of juvenile hemochromatosis (JH). Blood 2007;109:362–4.ArticlePubMedPDF
- 19. Nishio M, Endo T, Nakao S, Sato N, Koike T. Reversible cardiomyopathy due to secondary hemochromatosis with multitransfusions for severe aplastic anemia after successful non-myeloablative stem cell transplantation. Int J Cardiol 2008;127:400–1.ArticlePubMed
- 20. Kiguchi T, Ito Y, Kimura Y, Ohyashiki K. Restoration of cardiac function by an iron chelator, deferasirox, in a patient with aplastic anemia and cardiac iron overload. Int J Hematol 2009;89:546–8.ArticlePubMedPDF
- 21. Cappellini MD. Exjade(R) (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion. Ther Clin Risk Manag 2007;3:291–9.ArticlePubMedPMC
- 22. Cappellini MD, Taher A. Deferasirox (Exjade) for the treatment of iron overload. Acta Haematol 2009;122:165–73.ArticlePubMedPDF
- 23. Wood JC, Otto-Duessel M, Gonzalez I, Aguilar MI, Shimada H, Nick H, et al. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res 2006;148:272–80.ArticlePubMedPMC
- 24. Lee JW. Iron chelation therapy in the myelodysplastic syndromes and aplastic anemia: a review of experience in South Korea. Int J Hematol 2008;88:16–23.ArticlePubMedPMCPDF
- 25. Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et al. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol 2008;80:168–76.ArticlePubMedPMC
- 26. Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol 2008;88:24–9.ArticlePubMedPMCPDF
- 27. Piperno A. Classification and diagnosis of iron overload. Haematologica 1998;83:447–55.PubMed
- 28. Finch CA, Bellotti V, Stray S, Lipschitz DA, Cook JD, Pippard MJ, et al. Plasma ferritin determination as a diagnostic tool. West J Med 1986;145:657–63.PubMedPMC
- 29. Lombardo T, Tamburino C, Bartoloni G, Morrone ML, Frontini V, Italia F, et al. Cardiac iron overload in thalassemic patients: an endomyocardial biopsy study. Ann Hematol 1995;71:135–41.ArticlePubMedPDF
- 30. Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 1994;331:574–8.ArticlePubMed
- 31. Olivieri NF, Brittenham GM, Matsui D, Berkovitch M, Blendis LM, Cameron RG, et al. Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med 1995;332:918–22.ArticlePubMed
Citations
Citations to this article as recorded by




