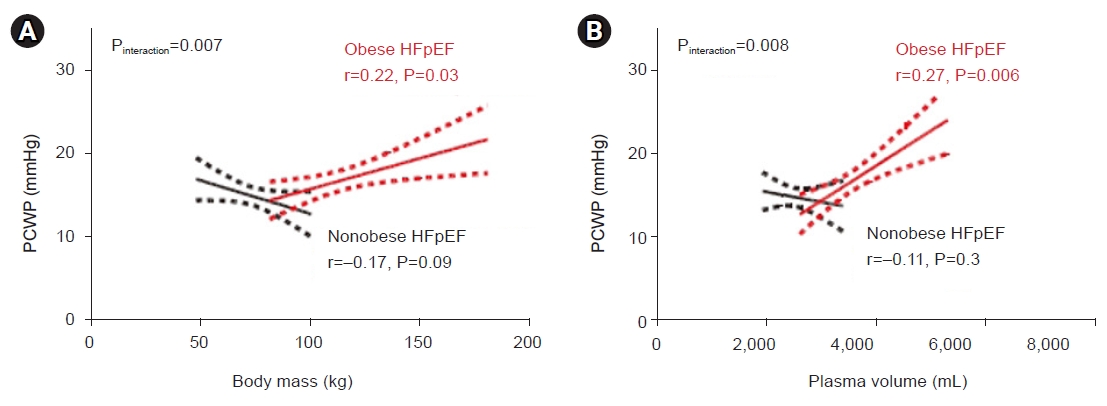
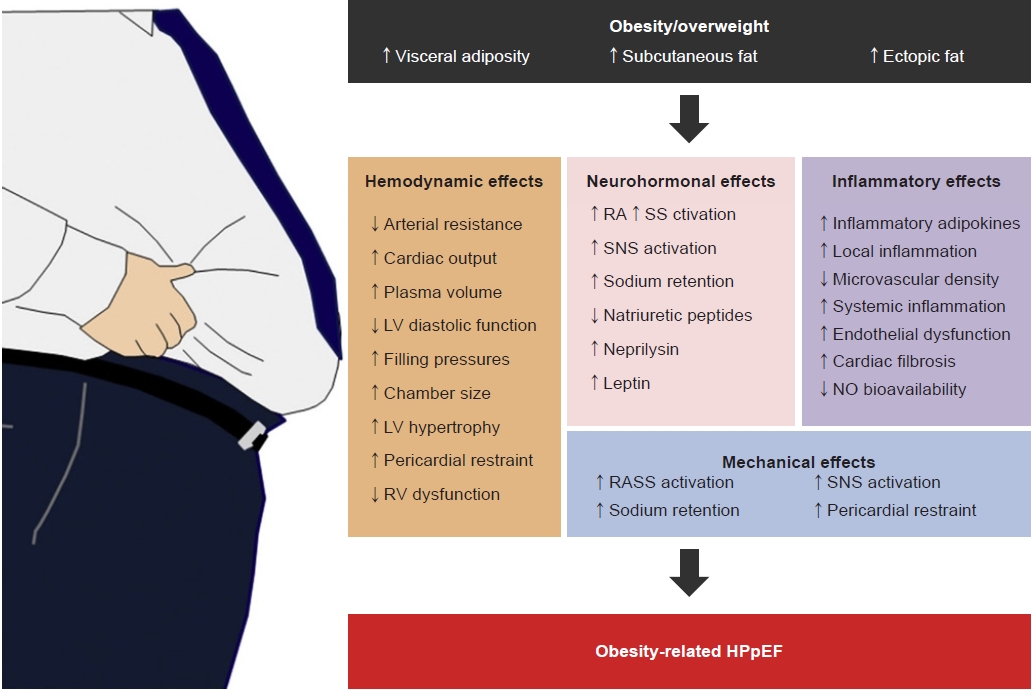
Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
Copyright © 2022 Korean Society of Cardiovascular Disease Prevention; Korean Society of Cardiovascular Pharmacotherapy.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ethical statements
Not applicable.
Conflicts of interest
The author has no conflicts of interest to declare.
Funding
None.