ABSTRACT
-
Background
- Since a sedentary lifestyle is considered a modifiable risk factor for cardiovascular disease (CVD), physical activity (PA) is recommended for type 2 diabetes mellitus (T2DM) patients to prevent CVD. We investigated the association between different levels of PA and the risk for CVD and all-cause mortality in patients with T2DM using nationwide data.
-
Methods
- We examined health examination data and claims records of 2,745,637 participants with T2DM at baseline from the Korean National Health Insurance Service who underwent health examinations between 2009 and 2012. We excluded subjects with a history of myocardial infarction or stroke. Each participant was asked to report their weekly PA levels according to three categories: vigorous, moderate, and walking. The incidence of CVD and death was analyzed until 2017.
-
Results
- The risk of CVD was lower in regular exercisers than in nonexercisers after adjusting for confounding variables. A dose-response trend was evident in the association between the degree of PA and CVD risk. All categories of PA were inversely associated with CVD risk and mortality. The reduction in CVD risk and all-cause mortality was more profound in patients aged ≥65 years.
-
Conclusions
- Augmenting PA might have positive effects on the prevention of CVD and all-cause death, especially in the elderly. The benefits of PA were consistently observed in various subgroups regardless of the presence of chronic conditions. Therefore, clinicians should encourage elderly patients with T2DM to increase their daily PA.
-
Keywords: Diabetes mellitus; Exercise; Cardiovascular disease; Physical activity; Prevention
INTRODUCTION
- The global prevalence of cardiovascular disease (CVD) among patients with type 2 diabetes mellitus (T2DM) is estimated at approximately 32.2% [1]. Despite advances in medicine, CVD remains a major health problem worldwide [2,3]. According to a brief report by Park et al. [4], the hospitalization rates owing to major cardiovascular complications and mortality among patients with T2DM have decreased because of improvements in medical treatment. However, the increase in the number of people with diabetes in aging societies, including South Korea, has made CVD a major contributor to mortality. Along with medical treatment for other risk factors of CVD, such as hypertension, interventions for other modifiable risk factors related to lifestyle are important for the primary prevention of CVD.
- Since a sedentary lifestyle is considered a modifiable risk factor for CVD, several meta-analysis studies have examined the effects of daily physical activity (PA) on CVD prevention. Sofi et al. [5] conducted a meta-analysis of 26 studies reporting protection against coronary heart disease in individuals with moderate or high levels of PA. Another meta-analysis, including 44 prospective cohort studies, demonstrated that PA had a negative linear correlation with the risk of cardiovascular mortality [6]. Patients with diabetes are at high risk of CVD; therefore, based on previous research, many guidelines have emphasized lifestyle modifications, including the promotion of PA, to lower the incidence of CVD. Most guidelines suggest that individuals should engage in a minimum of 150 min/wk of moderate-intensity PA for health benefits [7–9].
- However, there are discrepancies between guidelines and real-world applications in clinical practice. With the extended life expectancy in aging societies, an increasing number of elderly patients with T2DM will be unable to perform exercise of moderate to vigorous intensity owing to frailty and multiple comorbidities [10]. To date, no consensus exists on appropriate PA interventions in different stages of life, especially for elderly patients with T2DM. Moreover, most guidelines strongly recommend moderate to vigorous PA for the general population, whereas the benefits of light PA, such as slow walking, are rarely mentioned as key recommendations. Unfortunately, more than 60% of adults in the United States population fail to meet PA recommendations in terms of intensity and frequency and have a sedentary lifestyle [11]. According to a study investigating trends in adherence rates to PA guidelines in the United States [12], it is difficult to implement lifestyle modifications in the face of unrealistic PA recommendations that are challenging to fulfill in daily life. Therefore, feasible PA guidelines for patients with T2DM and further research on whether PA below the current recommendations for all age groups can prevent CVD and all-cause death are required. In this study, we investigated the association between different levels of PA and the risk for CVD and all-cause mortality in patients with T2DM using nationwide data.
METHODS
- Ethical statement
- This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital of Korea (No. KBSMC 2021-04-049). Participants who underwent national health checkup examinations provided written informed consent for the use of their data for research purposes. All personal information was deleted, and only de-identified data were included in the analysis.
- Source of data
- This study analyzed data from the Korean National Health Insurance Service (NHIS) and claims database. The NHIS is a public health insurance program that covers 97.1% of the Korean population. In Korea, the NHIS is the only healthcare insurer and is managed by the government. The NHIS includes an eligibility database (with data such as age, sex, socioeconomic variables, type of eligibility, and household income level); a medical treatment claims database (compiled based on medical bills that were claimed by medical service providers for medical expenses); a health examination database (including results of biennial general health examinations and questionnaires on lifestyle and behavior); a medical care institution database (including types of medical care institutions, location, equipment, and the number of physicians); and a death register [13]. For this study, we used the general health examination data and NHIS healthcare utilization data, including records on inpatient usage (diagnoses and procedures received), prescription records (drug codes, days prescribed, and daily dosage), and all-cause mortality.
- Study population
- This study included adults aged >20 years who underwent general health screening examinations. We selected participants who received a health examination between 2009 and 2012 and identified participants with T2DM at the baseline examination (n=2,745,637). A diagnosis of T2DM was defined according to the following criteria: (1) according to the International Classification of Diseases, 10th revision, clinical modification (ICD-10-CM) codes E11, E12, E13, or E14 and claims for at least one oral antidiabetic agent or insulin at baseline or (2) a fasting glucose level of ≥126 mg/dL (obtained from the health examination database). Among these individuals, subjects with missing data were excluded (n=94,423). Among the remaining subjects, we excluded 112,649 participants with a history of myocardial infarction (MI) and 254,287 participants with a history of stroke before the index date to ensure that all diagnoses of MI and stroke were newly made. Finally, 2,284,278 participants were included in the analysis. Fig. S1 shows the process used to select the study participants.
- Anthropometric and laboratory measurements
- The general health examination included medical history taking, physical measurements, blood and urine sampling after 12 hours of fasting, and chest radiography. Data on health-related behavior and lifestyle were collected by administering a self-reported questionnaire, whereas physical measurements and serum biochemical parameters were obtained by trained staff. Body mass index (BMI) was defined as the weight (kg) of the patient divided by the square of their height (m2). All laboratory values, including fasting blood glucose, aspartate aminotransferase, alanine aminotransferase, and total cholesterol levels, were measured after 12 hours of fasting.
- Assessment of PA
- The level of PA was evaluated using a self-administered questionnaire provided by the NHIS. The questionnaire was a modified version of the International Physical Activity Questionnaire, developed by the World Health Organization. The questionnaire comprised questions on the individual’s exercise frequency and intensity during the past 7 days. Regular exercise was defined as performing at least 30 minutes of moderate PA at least five times a week or at least 20 minutes of strenuous PA at least thrice a week. Each participant was asked to report their weekly PA levels according to the following three categories: vigorous PA (≥20 min/day; e.g., running, aerobics, or fast cycling at least thrice a week), moderate PA (≥30 min/day; e.g., brisk walking, bicycling at a usual speed, or gardening at least five times a week), and walking (≥30 min/day at least five times a week). Walking was defined as usual-pace walking for at least 10 minutes at a time [14]. The validity and reliability of the self-administered questionnaire on PA deployed in the NHIS cohort have been described in a previous study [15–17]. The English version of the self-administered questionnaire is presented in the Table S1.
- Definition of comorbidities
- Hypertension was defined according to the presence of at least one claim per year for the prescription of antihypertensive agents, under ICD-10-CM codes I10 through I15, or a systolic/diastolic blood pressure of ≥140/90 mmHg [18,19]. The presence of dyslipidemia was defined according to the presence of at least one claim per year for the prescription of antihyperlipidemic agents under ICD-10 code E78 or a total cholesterol level of ≥240 mg/dL [18,20]. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (GFR) of <60 mL/min [21].
- Outcome assessment
- CVD was defined as including MI and stroke. MI was defined by the occurrence of hospitalization with diagnostic codes I21 and I22, while stroke was defined by the presence of ICD-10-CM codes I63 and I64, as well as a history of hospitalization with claims for brain magnetic resonance imaging or brain computed tomography [19]. The incidence of CVD and death was analyzed using claims data from January 1, 2010, to December 31, 2017, or until the date of death, whichever occurred first.
- Statistical analysis
- Continuous variables are presented as mean±standard deviation, and categorical variables are expressed as percentages. The clinical characteristics of the participants were compared using one-way analysis of variance for continuous variables and the chi-square test for categorical variables. The incidence of MI, stroke, CVD, and death was presented as rates per 1,000 person-years. We defined the nonexerciser group as the reference group and conducted Cox proportional-hazards regression analysis with 95% confidence intervals (CIs) for incident CVD and mortality according to the levels of PA. The adjusted hazard ratio (aHR) was calculated after adjusting for the following variables: age, sex, smoking, alcohol consumption, household income, BMI, hypertension, dyslipidemia, GFR, fasting blood glucose, insulin use, duration of diabetes (≥5 years), and two or more oral hypoglycemic agents (OHAs).
- For the subgroup analysis, we stratified the participants by age (<40 years, <65 years, or >65 years), sex (male or female), duration of T2DM (≥5 years), insulin use, a combination of two or more OHAs, and CKD (yes or no). All reported P-values were two-tailed, and P<0.05 were considered statistically significant. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA) and R ver. 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org;).
RESULTS
- Baseline characteristics
- Participants’ characteristics are presented in Table 1. Of the 151,811 patients who developed CVD, the mean age was 63.8±10.8 years, and 90,866 of the patients (59.9%) were men. Patients who developed CVD more frequently had comorbidities such as hypertension and dyslipidemia than those without CVD (68.4% vs. 52.3% and 43.3% vs. 39.1%, respectively). Patients who developed CVD during the follow-up period were more likely to have unfavorable lipid profiles, a longer duration of diabetes (≥5 years, 41.7% vs. 27.0%), a higher percentage of insulin users (9.9% vs. 5.1%), and a combination of OHAs (≥2 OHAs, 28.5% vs. 23.6%). The proportion of regular exercisers was lower among patients who developed CVD (18.9% vs. 20.9%).
- Risk for CVD and mortality according to the dose of PA
- Multivariable-adjusted Cox proportional hazard analysis of CVD, stroke, MI, and all-cause mortality according to the dose of PA are presented in Table 2. The risk of CVD, MI, and stroke was lower in regular exercisers than in nonexercisers after adjusting for confounding variables such as smoking and alcohol consumption (aHR, 0.85 for CVD; aHR, 0.87 for MI; aHR, 0.84 for stroke; P<0.001). Regarding the association between the dose of PA and CVD risk, a dose-response trend was evident. All categories of PA were inversely associated with CVD risk and mortality. Compared with their control groups, participants who engaged in vigorous PA were associated with the lowest risk of CVD and all-cause mortality (aHR, 0.84 for CVD; aHR, 0.80 for all-cause mortality). Patients who engaged in light-intensity PA, such as walking, also had a reduced risk of CVD and mortality.
- Subgroup analysis
- The favorable effects of PA on CVD prevention may vary depending on the presence of other risk factors associated with T2DM. Thus, we conducted a subgroup analysis as follows: we stratified the participants by age (<40 years, <65 years, or >65 years), sex (male or female), duration of T2DM (≥5 years), insulin use, a combination of two or more OHAs, and CKD (yes or no). We conducted a subgroup analysis to determine which individuals experience more benefits of PA in lowering cardiovascular risk. In each subgroup, we compared CVD and mortality risk according to whether individuals engaged in regular exercise, vigorous exercise, moderate exercise, or walking.
- Effects of regular exercise on CVD and all-cause mortality
- The reduction in CVD risk and all-cause mortality was more profound in patients aged ≥65 years (CVD: HR, 0.82; 95% CI, 0.80–0.83; MI: HR, 0.85, 95% CI, 0.82–0.87; stroke: HR, 0.80; 95% CI, 0.78–0.81; all-cause mortality: HR, 0.77; 95% CI, 0.76–0.78; all P-values for interaction <0.001). However, patients aged ≤40 years did not show a decreased risk of CVD (Fig. 1A), MI (Fig. 1B), stroke (Fig. 1C), and all-cause mortality (Fig. 1D). The risk of CVD, MI, and death demonstrated an interaction with the duration of T2DM that might be interpreted as suggesting greater benefits of regular exercise in patients with a longer duration of diabetes (CVD: HR, 0.83; 95% CI, 0.81–0.84; MI: HR, 0.83; 95% CI, 0.81–0.86; all-cause mortality: HR, 0.76; 95% CI, 0.75–0.78). Interestingly, insulin treatment or multiple combinations of OHAs did not affect the risk of CVD and all-cause mortality. Regarding the risk of stroke in regular exercisers, there were no significant interactions for sex, duration of T2DM, insulin use, the number of OHAs, and the presence of CKD.
- Effects of vigorous PA on CVD and all-cause mortality
- The risk of CVD (Fig. 2A), MI (Fig. 2B), stroke (Fig. 2C), and all-cause mortality (Fig. 2D) showed a significant interaction with age, with a further risk reduction of CVD and death in patients aged ≥65 years who engaged in vigorous PA (CVD: HR, 0.82; 95% CI, 0.80–0.83; MI: HR, 0.85; 95% CI, 0.82–0.88; stroke: HR, 0.80; 95% CI, 0.78–0.82; all-cause mortality: HR, 0.79; 95% CI, 0.77–0.80; all P-values for interaction <0.05). The risk of CVD, MI, and death demonstrated an interaction with the duration of T2DM; however, an extended duration of diabetes did not affect the risk of stroke. Regarding mortality risk (Fig. 2D), we observed an interaction between sex and the presence of CKD (P-value for interaction <0.001). In the subgroup analysis performed among patients who engaged in vigorous PA, there was no interaction for CVD, MI, stroke, and all-cause mortality stratified by insulin use and multiple antidiabetic drugs.
- Effects of moderate PA on CVD and mortality
- Similar patterns were noted for CVD (Fig. 3A), MI (Fig. 3B), stroke (Fig. 3C), and death (Fig. 3D), and the results were largely consistent across patients who engaged in moderate-intensity PA. The overall favorable effects of moderate PA on the risk of CVD and all-cause mortality were similar across the stratified groups. The augmented benefits of lowering CVD risk and mortality were observed in patients aged ≥65 years who engaged in moderate levels of PA (CVD: HR, 0.84; 95% CI, 0.82–0.86; MI: HR, 0.86; 95% CI, 0.83–0.90; stroke: HR, 0.83; 95% CI, 0.80–0.85; all-cause mortality: HR, 0.80; 95% CI 0.78–0.81; all P-values for interaction <0.05). Interestingly, only patients with moderate PA had an interaction between the risk of stroke and the number of OHAs (HR, 0.82; 95% CI, 0.79–0.86; P-value for interaction <0.001). Regarding mortality risk (Fig. 3D), we observed additional interactions for sex, T2DM duration, and the presence of CKD (P-value for interaction <0.001).
- Effects of walking on CVD and mortality
- The overall favorable effects of walking on the risk of CVD and all-cause mortality were similar in the subgroup analysis. The risk of CVD (Fig. 4A), MI (Fig. 4B), stroke (Fig. 4C), and all-cause mortality (Fig. 4D) showed a significant interaction with age, with an additional risk reduction for CVD and death in the walking group among those aged ≥65 years. In patients who engaged in walking, the mortality risk was associated with age, sex, T2DM duration, insulin use, and the presence of CKD. Patients with T2DM receiving insulin therapy might receive moderate benefits from walking on all-cause mortality (HR, 0.85; 95% CI, 0.82–0.87; P-value for interaction <0.001). The magnitude of the risk reduction for CVD, MI, stroke, and mortality was greater in women who engaged in walking (CVD: HR, 0.88; 95% CI, 0.86–0.90; MI: HR, 0.88; 95% CI, 0.85–0.90; stroke: HR, 0.88; 95% CI, 0.86–0.90; all-cause mortality: HR, 0.83; 95% CI, 0.81–0.84; all P-values for interaction <0.001).
DISCUSSION
- Several studies have confirmed that PA helps lower CVD risk and mortality [22–24]. However, previous studies had limitations with respect to their study populations, such as including only men [22,25] or women [24,26], or involving a low percentage of patients with diabetes [23]. Large-scale studies examining whether light exercise can lower the incidence of CVD and mortality among patients with T2DM, including the elderly, are relatively rare. Using nationwide population health screening data and claims data, our findings lend additional evidence to current guidelines that recommend any amount of PA, including low-intensity PA, may lead to substantial risk reduction in CVD and mortality among elderly patients with T2DM.
- In the current study, we observed the benefits of regular exercise in lowering the risk of CVD and mortality in nationwide cohorts with T2DM. We found that all intensities of PA may help lower the risk for CVD and all-cause death. We also found an inverse dose-response relationship in which a higher level of PA was associated with a lower risk of CVD and all-cause death, with the highest risk reduction found for vigorous exercise, followed in descending order by moderate exercise and walking. In the subgroup analysis, we observed greater benefits of all levels of PA in lowering CVD and all-cause death among those aged ≥65 years. We also observed greater benefits of walking among female patients with T2DM and patients with a longer duration of T2DM. We observed consistent associations of PA with lower CVD risk and lower mortality in T2DM patients with or without any risk factors (e.g., >5 years of diabetes, insulin treatment, multiple OHAs, and CKD).
- Through a subgroup analysis, we were able to identify groups that could experience greater benefits of exercise. The impact on women was more significant in our study, and similar results have been published in previous studies [27]. There are possible explanations for the greater reduction observed in women. First, women are more likely to spend time performing household tasks than men, thereby engaging in a higher level of “background” habitual PA [27]. Second, there might be sex-specific physiological responses and differences in metabolism during exercise [28–30]. Beyond physiological differences, there might be differences in exercise behaviors and related factors between men and women [31]. There may also be differences in reporting PA in self-reported questionnaires. However, there is conflicting evidence regarding this finding. The risk reduction related to exercise was more prominent in men in the Nord-Trondelag Health Study [32].
- Another interesting finding in our study was that more benefits of PA were seen among the elderly and patients with a longer duration of T2DM. Sarcopenic obesity, which is prevalent in the elderly, seems to be associated with CVD [33,34]. Sarcopenic obesity and metabolic disease appear to share common pathways; therefore, engaging in PA may play an important role in the prevention of CVD [35]. Individuals with T2DM usually receive education from professionals to maximize their self-management ability, which might have prompted those with a longer duration of diabetes to maintain a healthier diet and engage in higher levels of PA.
- In a cross-sectional study conducted in Taiwan, participants (especially women) who had lower self-rated health scores were more likely to exercise regularly [31]. Patients with a longer duration of T2DM might have considered themselves as having an unhealthy status, and this self-perception might have promoted health-related behaviors.
- There are possible mechanisms that explain the benefits of PA on CVD and mortality. In general, PA and exercise lower blood pressure, improve lipid profiles, increase muscle mass, and prevent obesity. PA promotes nitric oxide release, resulting in endothelium-dependent vasodilation [36]. PA can have anti-inflammatory effects by increasing plasma levels of interleukin (IL)-10, reducing inflammatory cytokines (e.g., IL-6, tumor necrosis factor-α), platelet-related inflammatory mediators, and peripheral markers of endothelial dysfunction [37,38].
- Our study has several limitations that should be considered when interpreting the results. First, information on the frequency and intensity of PA was based on a self-reported questionnaire, which was subject to recall bias. Self-reported questionnaires may provide a reliable approximation of PA at the population level [39]; however, we cannot completely rule out the possibility of bias. We also did not collect detailed information regarding the type of PA (i.e., resistance exercise, aerobic exercise, or both) via the survey. Second, we did not consider the class effects of medications, which might have potential effects on the development of CVD and mortality. Although we tried to minimize confounding effects by adjusting for the duration of diabetes, insulin use, or the number of OHAs, we could not completely rule out the possibility of residual bias. We were unable to obtain data on the participants’ prescribed antidiabetic drugs, especially those that might have potential favorable effects on cardiovascular outcomes (e.g., sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists); however, according to Ko et al. [40], metformin has been the most commonly used antidiabetic drug in Korea (80.4% in 2013), while sulfonylureas are the most commonly prescribed second-line agents after metformin. According to the literature regarding the prescription patterns for T2DM in Korea, sodium-glucose co-transporter-2 inhibitors were first introduced in Korea at the end of 2013 and have not been commonly prescribed in newly diagnosed patients owing to reimbursement restrictions [41,42]. Third, we did not consider changes in PA patterns, body weight or body composition, and other health-related behaviors, including medication adherence, smoking status, and alcohol consumption during the follow-up period. Additional studies using time-varying confounders should be conducted in the future. Fourth, there is a possibility of underestimation of the incidence of CVD. We analyzed claims data for the outcomes; if a patient failed to visit the hospital and died at home because of a heart attack, this case may have been included in all-cause mortality but not as a CVD outcome. In this observational study, we were unable to establish a causal relationship. In addition, the results of this study might not be generalizable to other races. Given these limitations, randomized controlled trials are required to determine the most effective PA regimen for patients with T2DM. However, owing to ethical concerns regarding study designs controlling PA in populations at a high risk of CVD, conducting randomized controlled trials on this issue would be impossible.
- Despite its possible biases and limitations, our study has several strengths. Few studies have suggested an appropriate level of PA for elderly patients with diabetes who have multiple complications. Previous studies have often been limited to certain sex or age groups, and there have been few large-scale studies including younger people aged >20 years and the elderly. An advantage of our research is that it included both young and old patients with T2DM, including people aged >20 years. Clinical information such as insulin treatment and the number of antidiabetic drugs are crucial in epidemiological studies; however, few studies have analyzed this information to date. This is a nationwide population study including claims data, and we conducted a subgroup analysis by dividing the subjects according to these clinical factors.
- Although the causal relationship between PA and the risk of CVD and mortality could not be entirely addressed here, our results suggest that augmenting PA, even slow walking, might have positive effects on the prevention of CVD and all-cause death, especially in the elderly. Elderly patients with a longer duration of T2DM are at increased risk for hypoglycemia and have multiple comorbidities. Therefore, the current PA guidelines should be revised for the elderly to meet appropriate health goals.
- In conclusion, the magnitude of risk reduction in CVD and mortality might be greater in elderly patients with T2DM, and the benefits of PA were consistently observed for the various subgroups regardless of chronic conditions. Therefore, clinicians should encourage patients with T2DM to increase their daily PA, even if it were from low levels such as walking. Age-specific, sex-specific, and individualized PA recommendations should be established.
Supplementary Material
Fig. S1. Selection of study participants. T2DM, type 2 diabetes mellitus. Table S1. The English version of the questionnaire provided by the National Health Insurance Service during general health screening examinations. Supplementary data are available at https://doi.org/10.36011/cpp.2022.4.e3.
ARTICLE INFORMATION
-
Ethical statement
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital of Korea (No. KBSMC 2021-04-049). Participants who underwent national health checkup examinations provided written informed consent for the use of their data for research purposes. All personal information was deleted, and only de-identified data were included in the analysis.
-
Conflicts of interest
The authors have no conflicts of interest to disclose.
-
Funding
None.
-
Author contributions
Conceptualization: IJ; Data curation: SJM, HK, SEP; Formal analysis: KDH; Investigation: IJ, KDH; Methodology: KDH; Project administration: EJR, WYL; Resources: KDH; Software: KDH; Supervision: EJR, WYL; Validation: KDH; Visualization: IJ, KDH; Writing–original draft: IJ; Writing–review&editing: IJ, EJR, WYL. All authors read and approved the final manuscript.
-
Acknowledgments
The authors acknowledge the efforts of the Department of R&D Management at Kangbuk Samsung Hospital, Korea for editing the figures and tables. The authors would like to thank the National Health Insurance Service for their cooperation.
Fig. 1.Effects of regular exercise on (A) cardiovascular disease, (B) myocardial infarction, (C) ischemic stroke, and (D) all-cause mortality. HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
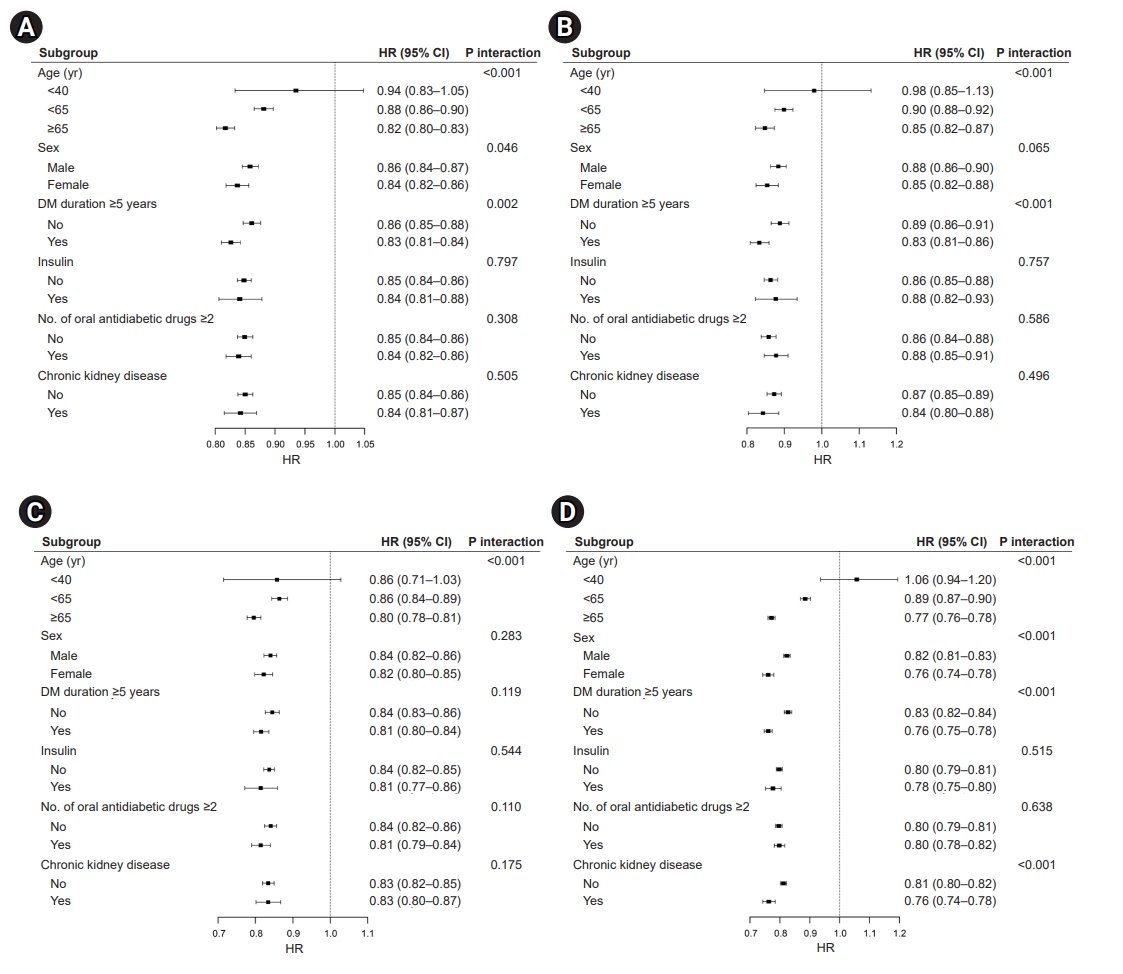
Fig. 2.Effects of vigorous physical activity on (A) cardiovascular disease, (B) myocardial infarction, (C) ischemic stroke, and (D) all-cause mortality. HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
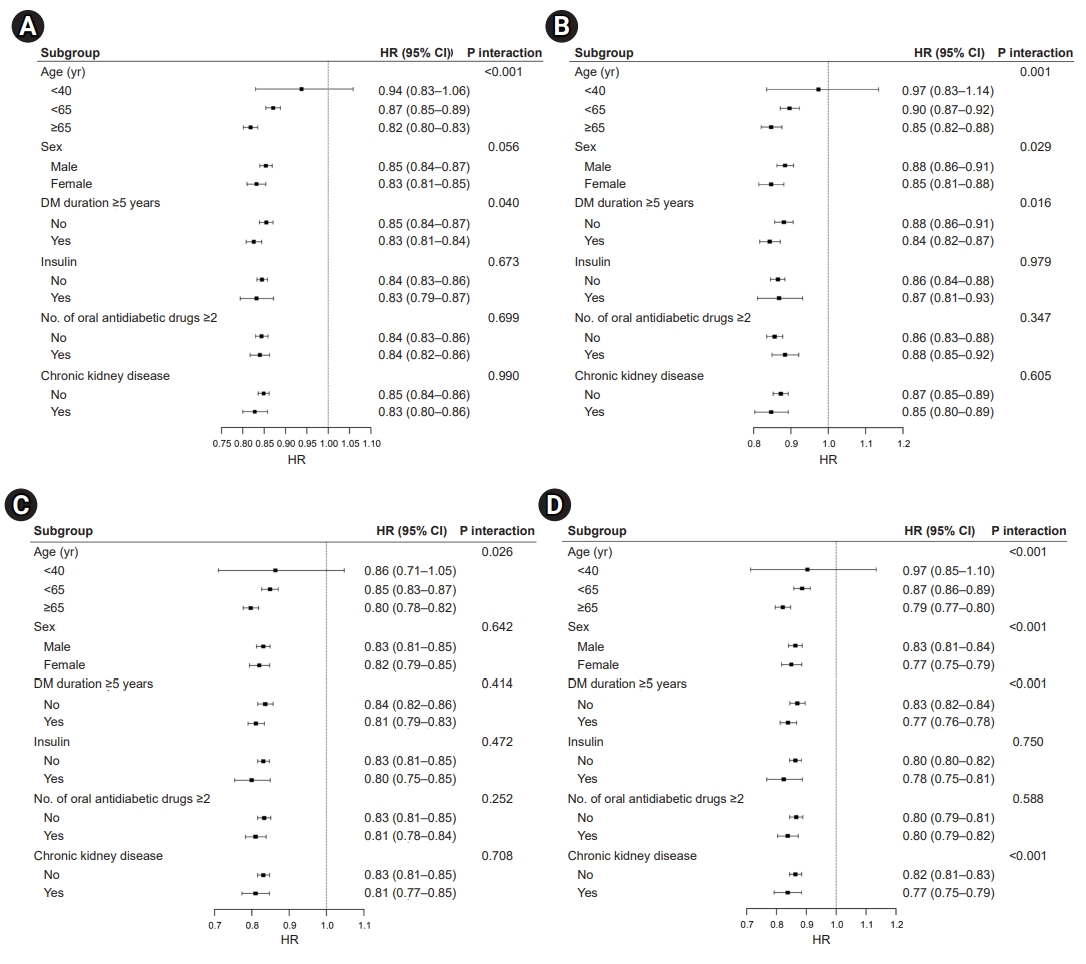
Fig. 3.Effects of moderate physical activity on (A) cardiovascular disease, (B) myocardial infarction (C) ischemic stroke, and (D) all-cause mortality. HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
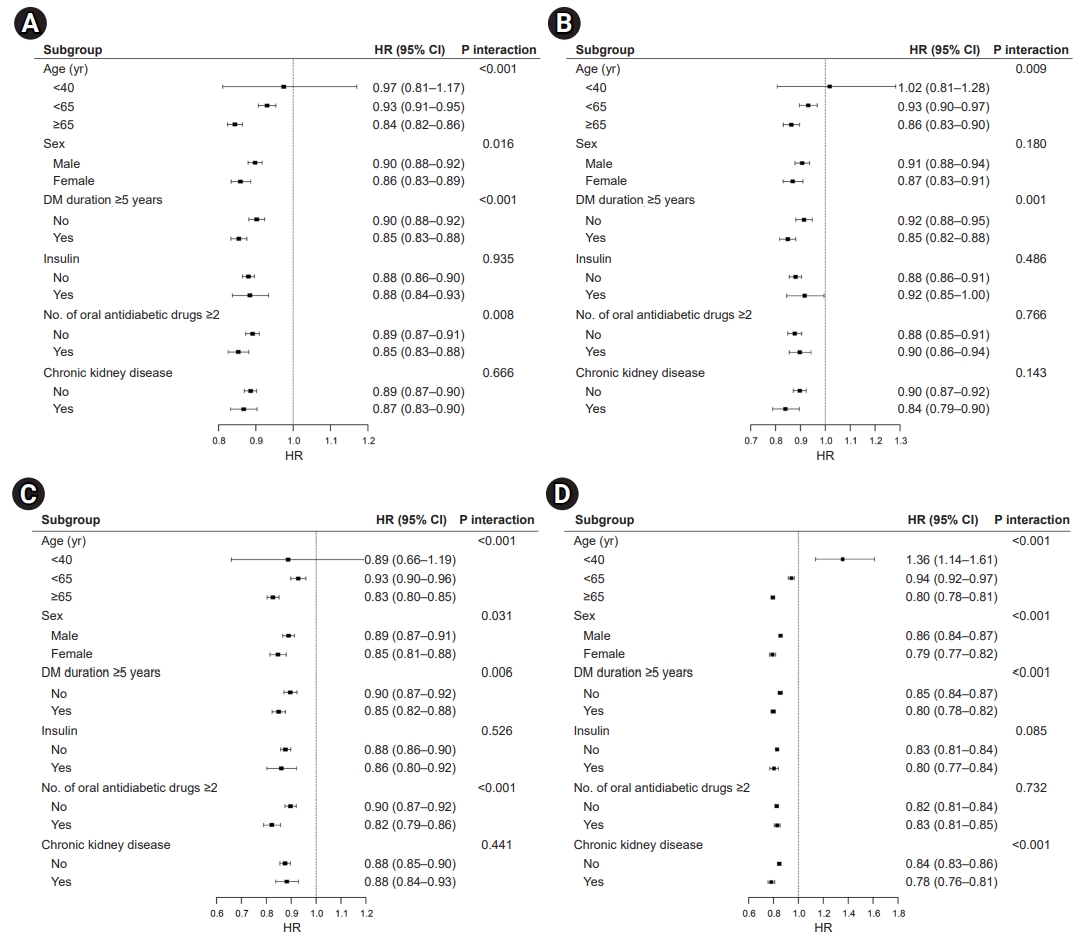
Fig. 4.Effects of walking on (A) cardiovascular disease, (B) myocardial infarction, (C) ischemic stroke, and (D) all-cause mortality. HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus.
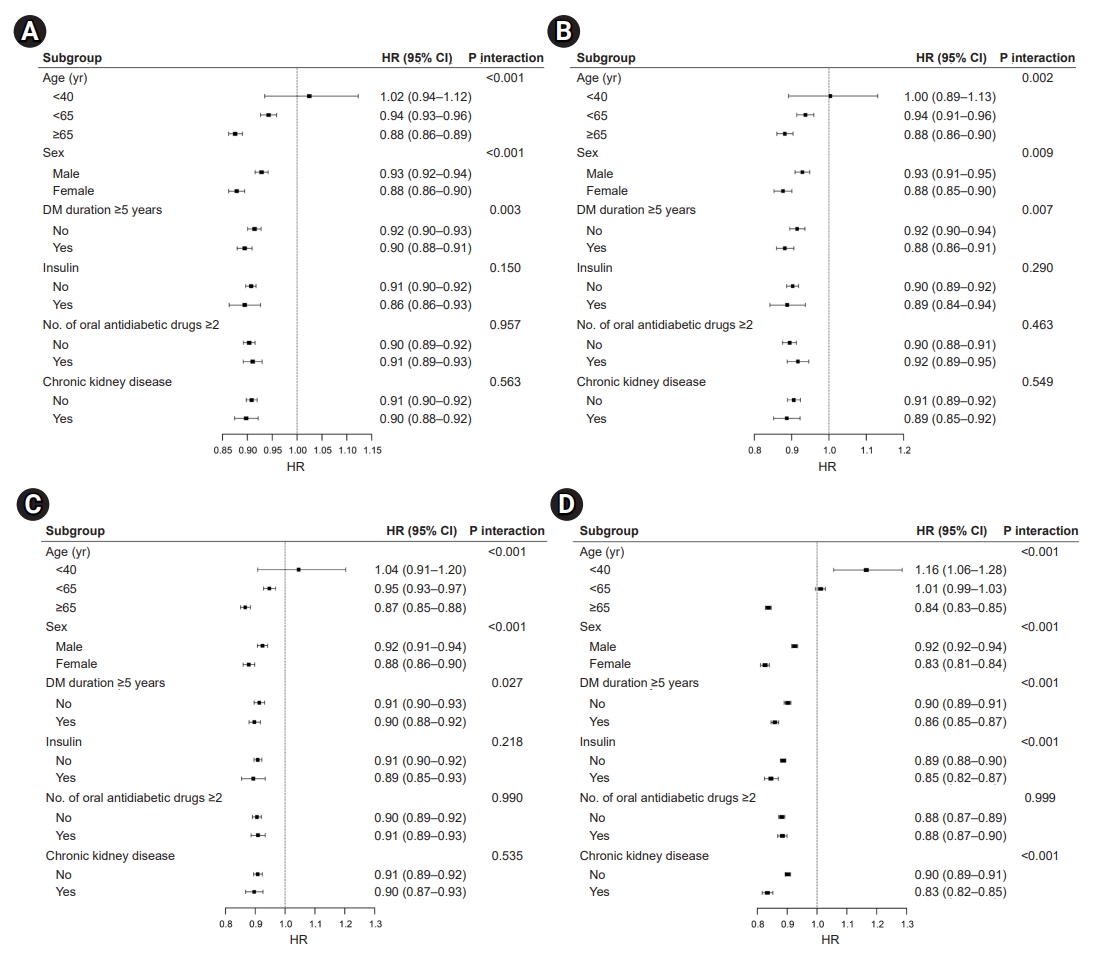
Table 1.Baseline characteristics of participants
|
Characteristic |
Total (n=2,284,278) |
Cardiovascular disease
|
P-value |
|
No (n=2,132,467) |
Yes (n=151,811) |
|
Age (yr) |
56.15±12.24 |
55.60±12.15 |
63.81±10.83 |
<0.001 |
|
Sex |
|
|
|
<0.001 |
|
Male |
1,400,555 (61.31) |
1,309,689 (61.42) |
90,866 (59.85) |
|
|
Female |
883,723 (38.69) |
822,778 (38.58) |
60,945 (40.15) |
|
|
Household income (lowest 20%) |
439,368 (19.23) |
407,957 (19.13) |
31,411 (20.69) |
<0.001 |
|
Hypertension |
1,219,281 (53.38) |
1,115,479 (52.31) |
103,802 (68.38) |
<0.001 |
|
Dyslipidemia |
899,389 (39.37) |
833,694 (39.10) |
65,695 (43.27) |
<0.001 |
|
Smoking status |
|
|
|
<0.001 |
|
Never smoker |
1,239,385 (54.26) |
1,154,144 (54.12) |
85,241 (56.15) |
|
|
Former smoker |
418,974 (18.34) |
394,500 (18.50) |
24,474 (16.12) |
|
|
Current smoker |
625,919 (27.40) |
583,823 (27.38) |
42,096 (27.73) |
|
|
Alcohol drinking |
|
|
|
<0.001 |
|
Never |
1,251,634 (54.79) |
1,154,729 (54.15) |
96,905 (63.83) |
|
|
Mild (<30 g/day) |
789,400 (34.56) |
748,537 (35.10) |
40,863 (26.92) |
|
|
Heavy (≥30 g/day) |
243,244 (10.65) |
229,201 (10.75) |
14,043 (9.25) |
|
|
Regular exercise |
473,410 (20.72) |
444,798 (20.86) |
28,612 (18.85) |
<0.001 |
|
DM duration ≥5 years |
638,885 (27.97) |
575,591 (26.99) |
63,294 (41.69) |
<0.001 |
|
Use of insulin |
122,785 (5.38) |
107,688 (5.05) |
15,097 (9.94) |
<0.001 |
|
Oral antidiabetic drugs ≥2 |
546,062 (23.91) |
502,847 (23.58) |
43,215 (28.47) |
<0.001 |
|
Body weight (kg) |
66.73±12.00 |
66.92±12.03 |
64.04±11.31 |
<0.001 |
|
Body mass index (kg/m2) |
25.07±3.71 |
25.09±3.74 |
24.75±3.35 |
<0.001 |
|
Waist circumference (cm) |
85.28±8.89 |
85.24±8.91 |
85.89±8.51 |
<0.001 |
|
Glomerular filtration rate |
86.03±36.37 |
86.42±36.43 |
80.54±34.96 |
<0.001 |
|
Fasting blood glucose (mg/dL) |
145.95±47.04 |
145.77±46.41 |
148.40±55.02 |
<0.001 |
|
SBP (mmHg) |
128.87±15.77 |
128.65±15.64 |
131.91±17.21 |
<0.001 |
|
DBP (mmHg) |
79.19±10.27 |
79.16±10.23 |
79.65±10.83 |
<0.001 |
|
Total cholesterol (mg/dL) |
198.45±46.21 |
198.36±46.21 |
199.66±46.28 |
<0.001 |
|
HDL-C |
52.46±29.99 |
52.54±30.04 |
51.42±29.20 |
<0.001 |
|
LDL-C |
113.8±86.17 |
113.68±86.24 |
115.55±85.13 |
<0.001 |
|
Triglyceridea)
|
147.21 (147.1–147.32) |
146.97 (146.85–147.08) |
150.70 (150.28–151.12) |
<0.001 |
|
AST (IU/L)a)
|
26.38 (26.37–26.40) |
26.43 (26.42–26.45) |
25.67 (25.61–25.73) |
<0.001 |
|
ALT (IU/L)a)
|
26.69 (26.67–26.71) |
26.86 (26.84–26.88) |
24.42 (24.35–24.49) |
<0.001 |
|
rGTP (IU/L)a)
|
37.71 (37.67–37.75) |
37.80 (37.76–37.85) |
36.46 (36.31–36.61) |
<0.001 |
Table 2.Risk for cardiovascular disease and mortality according to the dose of PA
|
Variable |
No. of patient |
Event |
Duration |
IR per 1,000 |
Modela)
|
|
1 |
2 |
3 |
4 |
|
Cardiovascular disease |
|
|
|
|
|
|
|
|
|
Regular exercise |
|
|
|
|
|
|
|
|
|
No |
1,810,868 |
123,199 |
13,836,283.94 |
8.90 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
473,410 |
28,612 |
3,691,757.17 |
7.75 |
0.87 (0.86–0.88) |
0.82 (0.81–0.83) |
0.85 (0.84–0.86) |
0.85 (0.84–0.86) |
|
Vigorous PA |
|
|
|
|
|
|
|
|
|
No |
1,895,076 |
129,120 |
14,485,597.02 |
8.91 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
389,202 |
22,691 |
3,042,444.09 |
7.46 |
0.83 (0.82–0.85) |
0.81 (0.80–0.83) |
0.85 (0.83–0.86) |
0.84 (0.83–0.86) |
|
Moderate PA |
|
|
|
|
|
|
|
|
|
No |
2,069,270 |
137,366 |
15,863,235.33 |
8.66 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
215,008 |
14,445 |
1,664,805.78 |
8.68 |
1.00 (0.98–1.02) |
0.86 (0.85–0.88) |
0.89 (0.87–0.90) |
0.88 (0.87–0.90) |
|
Walking |
|
|
|
|
|
|
|
|
|
No |
1,635,065 |
108,168 |
12,546,975.6 |
8.62 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
649,213 |
43,643 |
4,981,065.52 |
8.76 |
1.02 (1.01–1.03) |
0.90 (0.89–0.91) |
0.91 (0.90–0.92) |
0.91 (0.90–0.92) |
|
Myocardial infarction |
|
|
|
|
|
|
|
|
|
Regular exercise |
|
|
|
|
|
|
|
|
|
No |
1,810,868 |
52,745 |
14,075,488.55 |
3.75 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
473,410 |
12,480 |
3,747,353.55 |
3.33 |
0.88 (0.87–0.90) |
0.84 (0.82–0.85) |
0.87 (0.85–0.89) |
0.87 (0.85–0.88) |
|
Vigorous PA |
|
|
|
|
|
|
|
|
|
No |
1,895,076 |
55,246 |
1,476,512.04 |
3.75 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
389,202 |
9,979 |
3,086,330.05 |
3.23 |
0.86 (0.84–0.88) |
0.83 (0.82–0.85) |
0.87 (0.85–0.89) |
0.87 (0.85–0.88) |
|
Moderate PA |
|
|
|
|
|
|
|
|
|
No |
2,069,270 |
59,064 |
16,129,428.57 |
3.66 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
215,008 |
6,161 |
1,693,413.53 |
3.64 |
0.99 (0.96–1.02) |
0.87 (0.84–0.89) |
0.89 (0.87–0.92) |
0.89 (0.86–0.91) |
|
Walking |
|
|
|
|
|
|
|
|
|
No |
1,635,065 |
46,772 |
12,756,839.73 |
3.67 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
649,213 |
18,453 |
5,066,002.37 |
3.64 |
0.99 (0.98–1.01) |
0.90 (0.88–0.91) |
0.91 (0.89–0.92) |
0.90 (0.89–0.92) |
|
Stroke |
|
|
|
|
|
|
|
|
|
Regular exercise |
|
|
|
|
|
|
|
|
|
No |
1,810,868 |
77,803 |
13,979,386.22 |
5.57 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
473,410 |
17,657 |
3,726,185.01 |
4.74 |
0.85 (0.84–0.86) |
0.81 (0.79–0.82) |
0.84 (0.82–0.85) |
0.84 (0.82–0.85) |
|
Vigorous PA |
|
|
|
|
|
|
|
|
|
No |
1,895,076 |
81,565 |
14,635,569.37 |
5.57 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
389,202 |
13,895 |
3,070,001.85 |
4.53 |
0.81 (0.80–0.83) |
0.80 (0.79–0.82) |
0.83 (0.81–0.84) |
0.83 (0.81–0.84) |
|
Moderate PA |
|
|
|
|
|
|
|
|
|
No |
2,069,270 |
86,376 |
16,023,993.87 |
5.39 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
215,008 |
9,084 |
1,681,577.35 |
5.40 |
1.00 (0.98–1.02) |
0.86 (0.84–0.88) |
0.88 (0.86–0.90) |
0.88 (0.86–0.90) |
|
Walking |
|
|
|
|
|
|
|
|
|
No |
1,635,065 |
67,818 |
12,674,844.18 |
5.35 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
649,213 |
27,642 |
5,030,727.04 |
5.49 |
1.03 (1.01–1.04) |
0.90 (0.89–0.91) |
0.91 (0.90–0.92) |
0.91 (0.89–0.92) |
|
Death |
|
|
|
|
|
|
|
|
|
Regular exercise |
|
|
|
|
|
|
|
|
|
No |
1,810,868 |
162,459 |
14,233,226.29 |
11.41 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
473,410 |
35,975 |
3,784,834.50 |
9.51 |
0.83 (0.82–0.84) |
0.76 (0.75–0.77) |
0.79 (0.78–0.80) |
0.80 (0.79–0.81) |
|
Vigorous PA |
|
|
|
|
|
|
|
|
|
No |
1,895,076 |
170,078 |
14,901,819.81 |
11.41 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
389,202 |
28,356 |
3,116,240.98 |
9.10 |
0.79 (0.78–0.80) |
0.76 (0.75–0.77) |
0.79 (0.78–0.81) |
0.80 (0.79–0.81) |
|
Moderate PA |
|
|
|
|
|
|
|
|
|
No |
2,069,270 |
179,636 |
16,306,286.74 |
11.02 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
215,008 |
18,798 |
1,711,774.05 |
10.98 |
0.99 (0.98–1.01) |
0.80 (0.79–0.81) |
0.83 (0.82–0.84) |
0.83 (0.82–0.84) |
|
Walking |
|
|
|
|
|
|
|
|
|
No |
1,635,065 |
139,938 |
12,897,567.65 |
10.85 |
1 (reference) |
1 (reference) |
1 (reference) |
1 (reference) |
|
Yes |
649,213 |
58,496 |
5,120,493.14 |
11.42 |
1.05 (1.04–1.06) |
0.88 (0.87–0.89) |
0.89 (0.88–0.90) |
0.88 (0.87–0.89) |
REFERENCES
- 1. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. ArticlePubMedPMC
- 2. Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015;201 Suppl 1:S1–7.ArticlePubMed
- 3. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiological update. Eur Heart J 2013;34:3028–34.ArticlePubMed
- 4. Park JH, Ha KH, Kim BY, Lee JH, Kim DJ. Trends in cardiovascular complications and mortality among patients with diabetes in South Korea. Diabetes Metab J 2021;45:120–4.ArticlePubMed
- 5. Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil 2008;15:247–57.ArticlePubMed
- 6. Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prev Cardiol 2018;25:1864–72.ArticlePubMed
- 7. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2019;74:e177–232.ArticlePubMedPMC
- 8. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323.PubMed
- 9. Singh R, Pattisapu A, Emery MS. US Physical Activity Guidelines: current state, impact and future directions. Trends Cardiovasc Med 2020;30:407–12.ArticlePubMed
- 10. Charvat H, Goto A, Goto M, Inoue M, Heianza Y, Arase Y, et al. Impact of population aging on trends in diabetes prevalence: a meta-regression analysis of 160,000 Japanese adults. J Diabetes Investig 2015;6:533–42.ArticlePubMedPMC
- 11. Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA 2019;321:1587–97.ArticlePubMedPMC
- 12. Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, Bao W. Trends in adherence to the physical activity guidelines for Americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw Open 2019;2:e197597.ArticlePubMedPMC
- 13. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol 2017;46:799–800.ArticlePubMed
- 14. Chun MY. Validity and reliability of Korean version of International Physical Activity Questionnaire Short Form in the elderly. Korean J Fam Med 2012;33:144–51.ArticlePubMedPMC
- 15. Jeong HG, Kim DY, Kang DW, Kim BJ, Kim CK, Kim Y, et al. Physical activity frequency and the risk of stroke: a nationwide cohort study in Korea. J Am Heart Assoc 2017;6:e005671.ArticlePubMedPMC
- 16. Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) Short Form. J Korean Acad Fam Med 2007;28:532–41.PDF
- 17. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011;8:115. ArticlePubMedPMC
- 18. Seo MH, Kim YH, Han K, Jung JH, Park YG, Lee SS, et al. Prevalence of obesity and incidence of obesity-related comorbidities in Koreans based on National Health Insurance Service health checkup data 2006-2015. J Obes Metab Syndr 2018;27:46–52.PubMedPMC
- 19. Kim MK, Han K, Koh ES, Kim ES, Lee MK, Nam GE, et al. Blood pressure and development of cardiovascular disease in Koreans with type 2 diabetes mellitus. Hypertension 2019;73:319–26.ArticlePubMed
- 20. Kim MK, Han K, Kim HS, Park YM, Kwon HS, Yoon KH, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J 2017;38:3560–6.ArticlePubMedPMC
- 21. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: modification of diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70.ArticlePubMed
- 22. Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med 1998;338:94–9.ArticlePubMed
- 23. Schnohr P, Scharling H, Jensen JS. Intensity versus duration of walking, impact on mortality: the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil 2007;14:72–8.ArticlePubMed
- 24. Rockhill B, Willett WC, Manson JE, Leitzmann MF, Stampfer MJ, Hunter DJ, et al. Physical activity and mortality: a prospective study among women. Am J Public Health 2001;91:578–83.ArticlePubMedPMC
- 25. Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation 2003;107:2435–9.ArticlePubMed
- 26. Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med 2001;134:96–105.ArticlePubMed
- 27. Brown WJ, McLaughlin D, Leung J, McCaul KA, Flicker L, Almeida OP, et al. Physical activity and all-cause mortality in older women and men. Br J Sports Med 2012;46:664–8.ArticlePubMed
- 28. Ruby BC, Coggan AR, Zderic TW. Gender differences in glucose kinetics and substrate oxidation during exercise near the lactate threshold. J Appl Physiol (1985) 2002;92:1125–32.ArticlePubMed
- 29. O’Toole ML. Gender differences in the cardiovascular response to exercise. Cardiovasc Clin 1989;19:17–33.PubMed
- 30. Tarnopolsky MA. Gender differences in metabolism; nutrition and supplements. J Sci Med Sport 2000;3:287–98.ArticlePubMed
- 31. Mao HY, Hsu HC, Lee SD. Gender differences in related influential factors of regular exercise behavior among people in Taiwan in 2007: a cross-sectional study. PLoS One 2020;15:e0228191.ArticlePubMedPMC
- 32. Wisloff U, Nilsen TI, Droyvold WB, Morkved S, Slordahl SA, Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? ‘The HUNT study, Norway’. Eur J Cardiovasc Prev Rehabil 2006;13:798–804.ArticlePubMed
- 33. Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS One 2013;8:e60119.ArticlePubMedPMC
- 34. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc 2014;62:253–60.ArticlePubMedPMC
- 35. Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr 2005;82:428–34.ArticlePubMed
- 36. Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, et al. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A 2004;101:8797–802.ArticlePubMedPMC
- 37. Nunes RB, Tonetto M, Machado N, Chazan M, Heck TG, Veiga AB, et al. Physical exercise improves plasmatic levels of IL-10, left ventricular end-diastolic pressure, and muscle lipid peroxidation in chronic heart failure rats. J Appl Physiol (1985) 2008;104:1641–7.ArticlePubMed
- 38. Conraads VM, Beckers P, Bosmans J, De Clerck LS, Stevens WJ, Vrints CJ, et al. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur Heart J 2002;23:1854–60.ArticlePubMed
- 39. Loney T, Standage M, Thompson D, Sebire SJ, Cumming S. Self-report vs. objectively assessed physical activity: which is right for public health? J Phys Act Health 2011;8:62–70.ArticlePubMed
- 40. Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e4018.ArticlePubMedPMC
- 41. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J 2018;42:93–100.ArticlePubMedPMC
- 42. Kim JY, Kim SJ, Nam CM, Moon KT, Park EC. Changes in prescription pattern, pharmaceutical expenditure and quality of care after introduction of reimbursement restriction in diabetes in Korea. Eur J Public Health 2018;28:209–14.ArticlePubMed
Citations
Citations to this article as recorded by

- Incentivised physical activity intervention promoting daily steps among university employees in the workplace through a team-based competition
Ayazullah Safi, Sanjoy Deb, Adam Kelly, Matthew Cole, Natalie Walker, Mohammed Gulrez Zariwala
Frontiers in Public Health.2024;[Epub] CrossRef - Effect of physical activity on incident atrial fibrillation in individuals with varying duration of diabetes: a nationwide population study
JungMin Choi, So‑Ryoung Lee, Eue-Keun Choi, Kyung-Yeon Lee, Hyo-Jeong Ahn, Soonil Kwon, Kyung‑Do Han, Seil Oh, Gregory Y. H. Lip
Cardiovascular Diabetology.2024;[Epub] CrossRef
 , Sun Joon Moon1
, Sun Joon Moon1 , Hyemi Kwon1
, Hyemi Kwon1 , Se Eun Park1
, Se Eun Park1 , Kyung-Do Han2
, Kyung-Do Han2 , Eun-Jung Rhee1
, Eun-Jung Rhee1 , Won-Young Lee1
, Won-Young Lee1




