 , Jong-Chan Youn, MD, PhD2
, Jong-Chan Youn, MD, PhD2 , Sang Hong Baek, MD, PhD2
, Sang Hong Baek, MD, PhD2
1Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
2Division of Cardiology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Copyright © 2020 Korean Society of Cardiovascular Disease Prevention; Korean Society of Cardiovascular Pharmacotherapy.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6005448). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Kim ES; Supervision: Youn JC; Validation: Baek SH; Writing - review & editing: Kim ES.
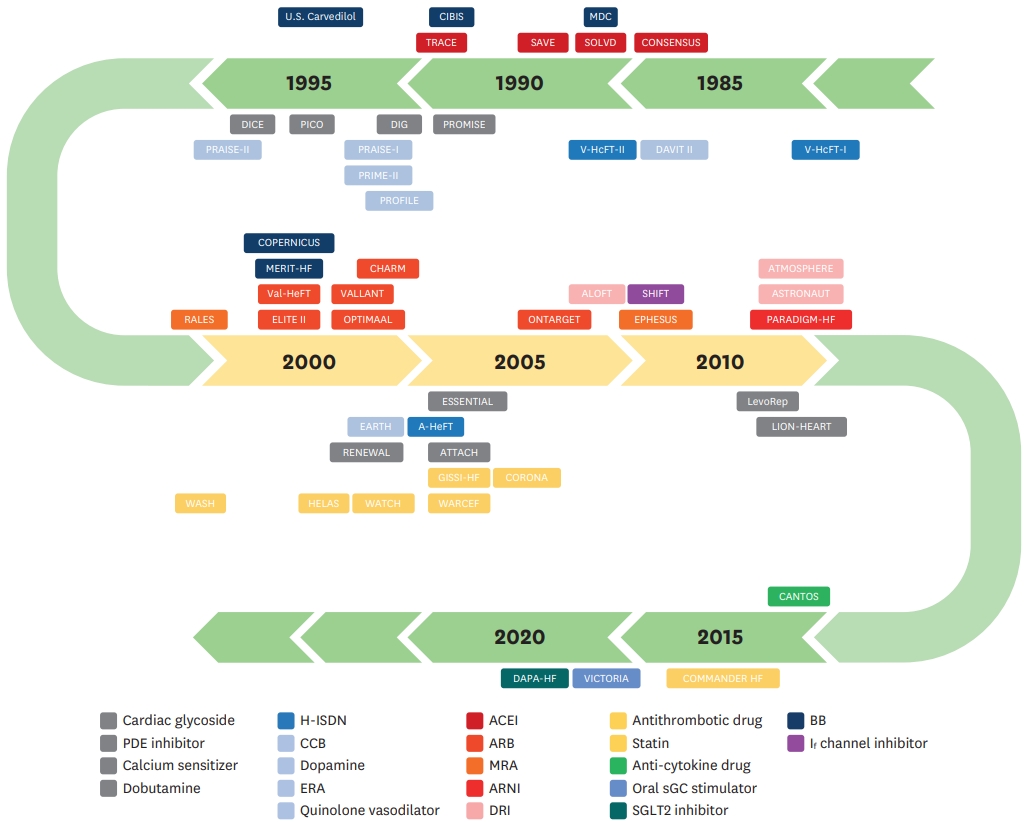
| Study | Year | Participants | Number | Intervention | Comparator | Follow-up | Primary endpoint | Mortality outcome | |
|---|---|---|---|---|---|---|---|---|---|
| ACEIs | |||||||||
| CONSENSUS13) | 1987 | NYHA class IV | 253 | Enalapril | Placebo | Mean 6 momths | All-cause mortality | • Mortality reduced by 40% (p=0.002) at the end of the study | |
| SOLVD14) | 1991 | EF ≤35% | 2,569 | Enalapril | Placebo | Mean 41 months | All-cause mortality | • Mortality reduced by 16% (95% CI, 5% to 26%; p=0.0036) | |
| • Death or hospitalization for CHF reduced by 26% (95% CI, 18% to 34%; p=0.0001) | |||||||||
| SAVE15) | 1992 | Post-MI EF ≤40% without overt HF | 2,231 | Captopril | Placebo | Mean 42 moths | All-cause mortality | • Mortality reduced by 19% (95% CI, 3% to 32%; p=0.0019) | |
| TRACE16) | 1995 | Post-MI EF ≤35% | 1,749 | Trandolapril | Placebo | Mean 36 months | All-cause mortality | • Mortality risk 0.78 (95% CI, 0.67 to 0.91; p=0.001) | |
| ARBs | |||||||||
| ELITE II17) | 2000 | EF ≤40%, NYHA class II–IV | 3,152 | Losartan | Captopril | Median 555 days | All-cause mortality | • No significant differences in all-cause mortality (HR, 1.13; 95.7% CI, 0.95 to 1.35; p=0.16) | |
| Val-HeFT18) | 2001 | NYHA class II–IV ACEIs (93%), BBs (35%) | 5,010 | Valsartan | Placebo | Mean 23 months | Mortality and a combined end point of mortality and morbidity | • Death from any cause (during entire trial) (RR, 1.02; 95% CI, 0.88 to 1.18; p=0.80) | |
| OPTIMAAL19) | 2002 | Post-MI EF <35%, NYHA class I–IV | 5,477 | Losartan | Captopril | Mean 2.7 years | All-cause mortality | • All-cause mortality (HR, 1.13; 95% CI, 0.99 to 1.28; p=0.07) | |
| CHARM-Alternative, Added20), 21) | 2003 | EF ≤40%, NYHA class II–IV | 4,576 | Candesartan | Placebo | Median 40 months | CV death or admission to hospital for management of worsening CHF | • CV death or hospitalization for CHF (HR, 0.82; 95% CI, 0.74 to 0.90; p<0.001) | |
| • All-cause mortality (HR, 0.88; 95% CI, 0.79 to 0.98; p=0.018) | |||||||||
| VALIANT22) | 2003 | Post-MI EF ≤35% | 14,703 | Valsartan | Captopril | Median 24.7 months | All-cause mortality | • All-cause-mortality valsartan vs. captopril (HR, 1.00; 97.5% CI, 0.90 to 1.11; p=0.98) | |
| Valsartan-Captopril | • All-cause-mortality valsartan-captopril vs. captopril (HR, 0.98; 97.5% CI, 0.89 to −1.09; p=0.73) | ||||||||
| ONTARGET23) | 2008 | High-risk patients with CVD or DM, but no HF | 25,620 | Telmisartan | Ramipril | Mean 56 months | Death from CV cause, myocardial infarction, stroke, or hospitalization for heart failure | • Telmisartan vs. ramipril primary outcome (RR, 1.01; 95% CI, 0.94 to 1.09) | |
| Telmisartan-Ramipril | • Telmisartan-ramipril vs. ramipril primary outcome (RR, 0.99; 95% CI, 0.92 to 1.07) | ||||||||
| • Telmisartan-ramipril vs. ramipril total number of discontinuations (RR, 1.20; p<0.0.01) | |||||||||
| BB | |||||||||
| MDC24) | 1993 | HF from idiopathic DCM, EF <40% | 383 | Metoprolol | Placebo | Mean 14 months | All-cause mortality or listing for cardiac transplantation | • Primary endpoint reduced by 34% (95% CI, −6 to 62%; p=0.058) | |
| CIBIS-I25) | 1994 | EF ≤40%, NYHA class III–IV | 641 | Bisoprolol | Placebo | Mean 1.9 years | All-cause mortality | • Mortality (RR, 0.8; 95% CI, 0.56 to 1.15; p=0.22) | |
| U.S. Carvedilol Heart Failure26) | 1996 | CHF EF ≤35% | 1,094 | Carvedilol | Placebo | Median 6.5 months | All-cause mortality | • Mortality reduced by 65% (95% CI, 39 to 80%; p<0.001) | |
| CIBIS-II27) | 1999 | EF ≤35%, NYHA class III–IV | 2,647 | Bisoprolol | Placebo | Mean 1.3 years | All-cause mortality | • All-cause mortality (HR, 0.66; 95% CI, 0.54 to 0.81; p<0.001) | |
| MERIT-HF28) | 1999 | EF ≤40%, NYHA class III–IV | 3,991 | Metoprolol CR/XL | Placebo | Mean 1 years | All-cause mortality | • All-cause mortality (HR, 0.66; 95% CI, 0.53 to 0.81; p<0.001) | |
| COPERNICUS29) | 2001 | EF ≤25%, NYHA class III–IV | 2,289 | Carvedilol | Placebo | Mean 10.4 months | All-cause mortality | • Mortality reduced by 35% (95% CI, 19 to 48%; p=0.0014) | |
| CAPRICORN30) | 2001 | Post-MI EF ≤40% | 1,959 | Carvedilol | Placebo | Mean 1.3 years | All-cause mortality or hospital admission for CV cause | • All-cause mortality (HR, 0.77; 95% CI, 0.60 to 0.98; p=0.03) | |
| MRA | |||||||||
| RALES31) | 1999 | EF ≤35% | 1,663 | Spironolactone | Placebo | Mean 24 months | All-cause mortality | • All-cause mortality (HR, 0.70; 95% CI, 0.60 to 0.82; p<0.001) | |
| EPHESUS32) | 2003 | EF ≤40% | 6,632 | Eplerenone | Placebo | Mean 16 months | All-cause mortality | • Death from any cause (RR, 0.85; 95% CI, 0.75 to 0.96; p=0.008) | |
| Death from CV causes or hospitalization for CV events | • Death from CV causes or hospitalization for CV events (RR, 0.87; 95% CI, 0.79 to 0.95; p=0.002) | ||||||||
| EPMPHASIS-HF33) | 2011 | EF ≤35% NYHA class II | 2,737 | Eplerenone | Placebo | Median 21 months | Death from CV causes or a first hospitalization for HF | • Primary outcome (HR, 0.63; 95% CI, 0.54 to 0.74; p<0.001) | |
| ISDN | |||||||||
| V-HeFT-I34) | 1986 | EF ≤45% | 642 | H-ISDN | Prazosin | Mean 2.3 years | All-cause mortality | • Mortality reduced by 34% (p=0.028) | |
| Placebo | |||||||||
| V-HeFT-II35) | 1991 | EF ≤45% | 804 | H-ISDN | Enalapril | Mean 2.3 years | All-cause mortality | • Mortality reduced by 28% in enalapril (p=0.0016) | |
| A-HeFT36) | 2004 | EF ≤45%, NYHA class III–IV | 1,050 | H-ISDN | Placebo | Mean 10 months | Death from any cause, a first hospitalization for HF, change in QOL | • Death from any cause (HR, 0.57; p=0.01) | |
| African-American | |||||||||
| If channel inhibitor | |||||||||
| SHIFT37) | 2010 | EF ≤35% | 6,558 | Ivabradine | Placebo | Mean 2.5 years | CV death or hospital admission for worsening HF | • Primary outcome (HR, 0.82; 95% CI, 0.75 to 0.90; p<0.0001) | |
| Sinus rhythm with heart rate >70 bpm | • CV death (HR, 0.91; 95% CI, 0.80 to 1.03; p=0.128) | ||||||||
| • Death from HF (HR, 0.74; 95% CI, 0.58 to 0.94; p=0.014) | |||||||||
| • All-cause-death (HR, 0.90; 95% CI, 0.80 to 1.02; p=0.092) | |||||||||
| ARNI | |||||||||
| OVERTURE38) | 2002 | EF ≤30%, NYHA class II–IV | 5,770 | Omapatrilat | Enalapril | Mean 14.5 months | All-cause mortality or hospitalization for HF | • Primary outcome (HR, 0.94; 95% CI, 0.86 to 1.03; p=0.187) | |
| PARADIGM-HF39) | 2014 | EF ≤40%, NYHA class II–IV | 8,442 | Sacubitril–Valsartan | Enalapril | Median 27 months | Death from CV causes or hospitalization for HF | • Primary outcome (HR, 0.80; 95% CI, 0.73 to 0.87; p<0.001) | |
| • All-cause death (HR, 0.84; 95% CI, 0.76 to 0.93; p<0.001) | |||||||||
| SGLT inhibitor | |||||||||
| DAPAHF40) | 2019 | EF ≤40%, NYHA class II–IV | 4,744 | Dapagliflozin | Placebo | Median 18.2 months | Worsening HF or CV death | • Primary outcome (HR, 0.74; 95% CI, 0.65 to 0.85; p<0.001) | |
| • CV death or HF hospitalization (HR, 0.75; 95% CI, 0.65 to 0.85; p<0.001) | |||||||||
| • All-cause death (HR, 0.83; 95% CI, 0.71 to 0.97; p=NA) | |||||||||
| Oral sGC stimulator | |||||||||
| VICTORIA41) | 2020 | EF ≤45%, NYHA class II–IV | 5,050 | Vericiguat | Placebo | Median 10.8 months | Death from CV causes or first hospitalization for HF | • Primary outcome (HR, 0.90; 95% CI, 0.82 to 0.98; p=0.02) | |
| • All-cause death or first hospitalization for HF (HR, 0.90; 95% CI, 0.83 to 0.98; p=0.02) | |||||||||
| • All-cause death (HR, 0.95; 95% CI, 0.84 to 1.07; p=0.38) | |||||||||
ACEI = angiotensin-converting-enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta-blocker; CHF = chronic heart failure; CI = confidence interval; CR/XL = controlled-release/extended-release preparation; CV = cardiovascular; CVD = cardiovascular disease; DCM = dilated cardiomyopathy; DM = diabetes mellitus, EF = ejection fraction; HF = heart failure; HR = hazard ratio; H-ISDN = hydralazine-isosorbide dinitrate; MI = myocardial infarction; NYHA = New York Heart Association; RR = relative risk; QOL = quality of life; sGC = soluble guanylate cyclase; SGLT = sodium-glucose co-transporter 2.
| Target | Study | Year | Participants | Number | Follow-up | Results | |
|---|---|---|---|---|---|---|---|
| Cardiostimulatory drugs | |||||||
| Dopamine | DICE55) | 1999 | NYHA class III–IV, EF ≤30% | 38 | 6 months | Intermittent low-dose dobutamine did not improve the functional status or mortality rate compared with placebo | |
| Amrinone | Massie et al.56) | 1985 | NYHA class III–IV | 99 | 12 weeks | Adverse reactions were significantly more frequent and more severe with amrinone, occurring in 83% of patients | |
| Milrinone | PROMISE57) | 1991 | NYHA class III–IV, EF ≤35% | 1,088 | Median6.1 months | There was a 28% increase in all-cause mortality with milrinone therapy compared with placebo | |
| Enoximone | ESSENTIAL58) | 2009 | NYHA class III–IV, EF ≤30% | 1,854 | Median 16.6 months | Composite of time to all-cause mortality or CV hospitalization did not differ compared with placebo | |
| Vesnarinone | Jay et al.59) | 1998 | NYHA class III–IV, EF ≤30% | 3,833 | Mean 9.5 months | Dose-dependent increase in mortality with vesnarinone compared with placebo | |
| Pimobendan | PICO60) | 1996 | NYHA class II–III, EF ≤45% | 317 | 24 weeks | In both pimobendan groups (2.5 and 5 mg) combined the hazard of death was 1.8 times higher compared with placebo | |
| Levosimendan | LevoRep61) | 2014 | NYHA class III–IV, EF ≤35% | 120 | 24 weeks | Pulsed infusions of levosimendan did not significantly improve functional capacity or quality of life compared with placebo | |
| Levosimendan | LION-HEART62) | 2018 | NYHA class III–IV, EF ≤35% | 69 | 12 weeks | Pulsed infusions of levosimendan significantly reduced NT-proBNP levels and risk of hospitalization | |
| Reduction of all-cause death was not statistically significant compared with placebo | |||||||
| Digoxin | DIG63) | 1997 | EF ≤45% | 3,397 | Mean 37 months | Reduced rate of hospitalization both overall and for worsening HF (RR, 0.72) compared with placebo | |
| Insignificant reduction in overall mortality (RR, 0.99; p=0.80) | |||||||
| Drugs with a vasodilatory effect | |||||||
| Nifedipine | Elkayam et al.64) | 1990 | NYHA class II–III, EF ≤40% | 28 | 8 weeks | Significantly higher incidence of clinical deterioration and worsening of CHF was observed with nifedipine alone or in combination with isosorbide dinitrate compared with placebo | |
| Verapamil | DAVIT II65) | 1990 | Post MI | 1775 (HF: 614) | Mean 16 months | Verapamil did not improve mortality or hospitalization for HF patients compared with placebo | |
| Diltiazem | Goldstein et al.66) | 1991 | Post MI, Baseline EF ≤40% | 623 | Mean 25 months | Diltiazem was associated with enhanced CHF after MI in patients with preceding LV dysfunction compared with placebo | |
| Amlodipine | PRAISE67) | 1996 | NYHA class III–IV, EF ≤30% | 1,153 | Median 13.8 months | No difference between the amlodipine and placebo groups in the occurrence of all-cause death and hospitalization for major CV events | |
| Amlodipine | PRAISE II68) | 2013 | NYHA class III–IV, EF ≤30% | 1,654 | Median 33 months | Amlodipine had no benefits on clinical outcomes for patients with HF, regardless of underlying coronary artery disease compared with placebo | |
| Nonischemic cardiomyopathy | |||||||
| Felodipine | V-HeFT III69) | 1997 | EF ≤45% | 450 | Mean 18 months | Trend for long-term beneficial effects in exercise tolerance and depression for quality of life | |
| Ibopamine | PRIME II70) | 1997 | NYHA class III–IV, EF ≤35% | 1,906 | Mean 347 days | Increased the risk of death among patients (RR 1.26, p=0.017) treated with ibopamine compared with placebo | |
| Darusentan | EARTH71) | 2004 | NYHA class II–IV, EF ≤35% | 642 | 24 weeks | No benefit in cardiac remodeling, clinical symptoms, or outcomes compared with placebo | |
| Bosentan | Packer et al.72) | 2017 | NYHA class III–IV, EF ≤35% | 1,613 | Median 1.5 years | No improvement in clinical outcomes and increased fluid retention within the first 2–4 weeks compared with placebo | |
| Flosequinan | Packer et al.73) | 2017 | NYHA class III–IV, EF ≤35% | 2,354 | Median 10 months | Increased risk of death in patients treated with flosequinan compared with placebo (HR 1.39, p=0.0006) | |
| RAAS modulators | |||||||
| Aliskiren | ALOFT74) | 2008 | NYHA class II–IV | 302 | 3 months | Reduced plasma BNP and urinary aldosterone with aliskiren | |
| Plasma BNP >100 pg/mL | No effect on blood pressure or biochemistry compared with placebo | ||||||
| Aliskiren | ASTRONAUT75) | 2013 | NYHA class II–IV, EF ≤30% | 1,615 | Median 11.3 months | No reduction in CV death or HF rehospitalization compared with placebo | |
| BNP ≥400 pg/mL or NT-proBNP ≥1,600 pg/mL NYHA class II–IV, EF ≤35% | No benefit of aliskiren added to enalapril but increased adverse events | ||||||
| Aliskiren | ATMOSPHERE76) | 2016 | BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL | 2,336 | Median 36.6 months | Aliskiren did not show noninferiority compared with enalapril | |
| Antithrombotic drugs | |||||||
| Aspirin | WASH77) | 2004 | EF ≤35% | 279 | Mean 27 month | No benefit in clinical outcome compared with placebo | |
| Warfarin | More patients in the aspirin group were hospitalized for worsening HF | ||||||
| Aspirin | HELAS78) | 2006 | EF <35% | 197 | Mean 21.9 months | No clinical benefit of aspirin or warfarin compared with placebo | |
| Warfarin | Ischemic heart disease (aspirin) | ||||||
| Dilated cardiomyopathy (warfarin) | |||||||
| Exclusion: Atrial fibrillation | |||||||
| Aspirin | WATCH79) | 2009 | NYHA class II–IV, EF ≤35% | 1,587 | Mean 1.9 years | No statistical difference between aspirin, clopidogrel, and warfarin on mortality risk | |
| Clopidogrel | Normal sinus rhythm | ||||||
| Warfarin | |||||||
| Aspirin | WARCEF80) | 2012 | EF ≤35% | 2,305 | Mean 3.5 years | No significant reduction on composite end point of ischemic stroke, intracerebral hemorrhage, or death from any cause between aspirin and warfarin | |
| Warfarin | Normal sinus rhythm | ||||||
| Rivaroxaban | COMMANDER HF81) | 2018 | EF ≤40% | 5,022 | Median 21.1 months | No significant reduction in the rate of death, myocardial infarction, or stroke compared with placebo | |
| BNP ≥200 pg/mL or NTproBNP ≥800 pg/mL | |||||||
| Exclusion: Atrial fibrillation | |||||||
| Statins | |||||||
| Rosuvastatin | CORONA82) | 2007 | NYHA class II–IV Age ≥60 years | 5,011 | Median 32.8 months | No reduction in mortality in the rosuvastatin group, but fewer hospitalization compared with placebo (p<0.001) | |
| Rosuvastatin | GISSI-HF83) | 2008 | NYHA class II–IV | 4,631 (EF ≤40%: 4,113) | Median 3.9 years | Rosuvastatin had no benefit on clinical outcomes | |
| including the subgroup with EF ≤40% compared with placebo | |||||||
| Anti-cytokine drugs | |||||||
| Infliximab | ATTACH84) | 2003 | NYHA class III–IV, EF ≤35% | 150 | 28 weeks | No clinical benefit, and higher dose of infliximab (10 mg/kg) increased the combined risk of death from any cause or hospitalization compared with placebo | |
| Etanercept | RENEWAL85) | 2004 | NYHA class II–IV, EF ≤30% | 1,123 (RECOVER) | 24 weeks | Etanercept had no effect on death or hospitalization due to CHF | |
| 925 (RENAISSANCE) | |||||||
| Canakinumab | CANTOS86) | 2019 | Prior MI | 10,061 (HF: 2,173) | Median 3.7 years | Dose-dependent reduction in hospitalization for HF and the composite of hospitalization for HF or Hrrelated mortality compared with placebo | |
| hsCRP ≥2 mg/L | |||||||
BNP = B-type natriuretic peptide; CHF = chronic heart failure; CV = cardiovascular; EF = ejection fraction; HF = heart failure; HR = hazard ratio; hsCRP = highsensitivity C-reactive protein; LV = left ventricular; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; RAAS = renin-angiotensin-aldosterone system; RR = relative risk.



 PubReader
PubReader ePub Link
ePub Link Cite
Cite